 Role of Biological Factors on the Survival of Escherichia coli, Salmonella paratyphi and Vibrio parahaemolyticus in a Tropical Estuary, India
Role of Biological Factors on the Survival of Escherichia coli, Salmonella paratyphi and Vibrio parahaemolyticus in a Tropical Estuary, India
Abhirosh C1, Sheeja KM2, Hatha AAM3, Sherin V4, Thomas AP5
1 School of EnvironmentalSciences, Mahatma Gandhi University, Priyadarsini Hills P.O., Kottayam686 560, Kerala, India.
2 School of Marine Sciences, Cochin Universityof Science and Technology, Cochin, Kerala, India
1 Correspondence: abhichandn@gmail.com
2 Correspondence: sheejakm@gmail.com
3 Correspondence: mohamedhatha@gmail.com
4 Correspondence: sherinannav@gmail.com
5 Correspondence: ambattu_ktm@sify.com
Key words: E. coli, S. paratyphi, V. parahaemolyticus, bacteriophages, predation, competition, survival, estuary, India.
Received 3 June 2009; revised 6 August 2009; accepted 12 September 2009.Published 15 October 2009. Available online 15 October 2009.
Summary
Microcosm studies have been carried out to evaluate the role of biological factors such as protozoan predation, bacteriophages andautochthonous bacterial competition on the survival of Escherichiacoli, Salmonella paratyphi and Vibrio parahaemolyticus in estuarine water at 20oC and 30oC. The role of protozoans was studied using raw estuarine water. The effect of competing autochthonous bacteria was studied in autoclaved estuarine water inoculated with heterotrophic bacteria isolated from the estuarine water. All the microcosms were inoculated with the above three microorganisms at a concentration of106-7 cfu/ml. Of the three factors protozoan predation was the major factor (followed by bacteriophages) influencing the mortality of the test organisms. Competition by autochthonous bacterial population had little effect on the test organisms. In cycloheximide treated and non-treated estuarine water, E. coli, S. paratyphi and V.parahaemolyticus showed more robust survival at 20oC than at 30oC.However, no significant (P>0.05) variation in the survival of the test organisms was observed in cycloheximide-treated, non-treated andin microcosm-inoculated with autochthonous bacteria. This information is important in order to understand self-purifying factors and their relative importance in the aquatic environments.
Article Outline
- Introduction
- Materials and Methods
- Survival Experiments
- Results
- Discussion
- Conclusion
- References
- Discussion With Reviewers
Introduction
The survival and persistence of allochthonous enteric microorganisms in the natural aquatic environment has been of interest to public health and microbial ecology (Catalao et al., 2000). There are various physico-chemical and biological factors involved in the disappearance of pollutant microorganisms in the aquatic environment (Abhirosh and Hatha, 2005). Among biological factors, grazing by protozoans (Hahn and Hofle, 2001)—especially nano-flagellates and ciliates—have been identified as a significant factor in modifying bacterial populations in aquatic ecosystems (Sherr and Sherr, 2002). The specific role of bacteriophages in the removal of bacterial communities has been reported (Fuhrman and Noble, 1995; Hennes and Simon, 1995; Duhamel et al., 2006). It has been also documented that competing autochthonous microbiota also affects the bacterial population (McCambridge and McMeekin, 1981; Le Guyader et al., 1991).
The presence of E. coli has been widely accepted as an indicator of fecal contamination and therefore as an indicator of the possible presence of pathogens of enteric origin. However, certain diarrhegenic serotypes such as enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enterohemorrhagic E. coli (EHEC), enteropathogenic E. coli (EPEC) enteroaggregative E. coli (EAggEC) and enteroadhesive E. coli (DAEC) have been reported as an emerging public health concern for children in developing countries (Kaper et al., 2004). The prevalence of these serotypes has been reported in tropical estuarine water (Hatha et al., 2004). Vibrios are an important human enteric pathogen and infections are frequently reported in coastal areas, apparently because of the high consumption of sea products and direct contact with estuarine waters (Marano et al., 2000). Salmonella spp are the etiologic agents of food-borne and water-borne pathogens causing Salmonellosis, which results in an annual occurrence of about 17 million cases worldwide (Kindhauser, 2003).
The removal kinetics of enteric pathogens in tropical estuarine water is scarce. Also, there is different opinion about the individual roles of protozoan predation, autochthonous bacterial competition and the effect of bacteriophages on the regulation of enteric bacterial populations in different aquatic environments. In our previous study we observed high prevalence of E. coli, S. paratyphi and V. parahaemolyticus in Vembanadu Lake (Abhirosh et al., 2008). Hence it is very important to study the factors affecting the survival of these organisms in estuarine water. There are no published data available on the survival of these organisms in Vembanadu lake, which is used for various recreational activities. Therefore, in the present investigation, microcosm studies have been carried out to evaluate the role of biological factors such as protozoan predation, bacteriophages and autochthonous bacterial competition on the survival of E. coli, S. paratyphi and V. parahaemolyticus in estuarine water.
Materials and Methods
Samples: The study has been carried out in water collected from Vembandu estuary (Fig.6) (9o35’N 76o25’E)which is an important Ramsar site in Kerala, South India. Water samples were collected (once in a month) in sterile plastic bottles and transported to the laboratory in an icebox and subjected to bacteriological examination within four hours of collection. The samples were processed for heterotrophic bacteria as per USEPA (1978).
Enumeration of Heterotrophic Bacteria (THB): Water samples were decimally diluted using sterile distilled water and shaken thoroughly to distribute the microorganisms in the water to the dilution blank. Appropriately diluted samples were plated on Tryptone Soya Agar (TSA) using spread plate technique. The inoculated plates were incubated at room temperature for 24-48 hrs. After incubation plates with countable range (30-300 colonies) were selected for counting (using a colony counter) and the bacterial load in the sample were expressed as total colony forming units (cfu) per ml of the water sample. Then morphologically different colonies were isolated and identified as various genera as per Bergey’s manual of determinative bacteriology (Buchanan and Gibbons, 1984). Protozoan were analyzed qualitatively and identified according to Batish (1992).
Survival Experiments
Test Organisms: Confirmed cultures of E. coli, S. paratyphi and V. parahaemolyticus isolated from Vembanadu Lake (Abhirosh et al., 2008) were maintained in the culture collection of the Microbiology Department of the School of Environmental Sciences. These were used for survival studies. Vibrio parahaemolyticus is an indigenous marine bacterium, which was selected because of its importance as a food-borne pathogen.
Preparation of the Inocula: The inocula were prepared as previously described by Abhirosh and Hatha (2005). Briefly, E. coli/ S. paratyphi/V. parahaemolyticus were grown in Tryptone Soya Broth (TSB) and incubated at 37oC for 24 hours. After incubation, the cells were concentrated by centrifugation at 3000 rpm for 15 minutes and washed twice with sterile isotonic saline. After the final wash, the cells were suspended in the same isotonic saline.
Microcosm: Water microcosms were prepared as previously described (Abhirosh and Hatha, 2005). 100 ml of estuarine water was taken in an Erlenmeyer flask (250 ml capacity) and was used as microcosms. Then individual microcosms were inoculated separately with E. coli/ S. paratyphi/ V. parahaemolyticus (approximately 107-8 cfu/ml). After inoculation the microcosms were mixed thoroughly for the uniform distribution of the bacteria in the microcosms. After that, all the microcosms were incubated (without agitation) at 20o and 30oC. The microcosms were covered with black paper to prevent light exposure. The following test solutions were used for the survival study.
Test Solutions: To find out the effect of various biological factors such as protozoan predation, bacteriophages, and competition from autochthonous bacteria, survival experiments were conducted in estuarine water treated with a eukaryotic inhibitor “cycloheximide” (treated) and without cycloheximide (non-treated) as follows.
Non-Treated Estuarine Water: This test solution was used to determine the effect of the self-contained biological factors such as protozoan and bacteriophages on the test organisms.
Treated Estuarine Water: This test solution was used to study the effect of bacteriophages alone on the test organisms. Protozoans were inhibited from the test solution by the addition of cycloheximide at a concentration of 500 mg/l (Davies et al., 1995).
Autoclaved Estuarine Water Inoculated with all Autochthonous Bacterial spp: This test solution was used to study the effect of competing autochthonous bacteria isolated from estuarine water acting together on the survival of the test organisms. One milliliter of washed cells (see section, preparation of inocula) of all the 8 bacterial species were added together into the test solution containing E. coli/ S. paratyphi/ V. parahaemolyticus in separate bottles. All the test solutions were incubated at 20oC and also at 30oC in order to find out the survival at low temperatures as the temperature went down to 20oC in winter as well as at a certain depth.
Autoclaved Estuarine Water Inoculated with all Autochthonous Bacterial spp: This test solution was used to study the effect of competing autochthonous bacteria isolated from estuarine water acting together on the survival of the test organisms. One milliliter of washed cells (see section, prepartion of inocula) of all the eight bacterial species were added together into the test solution containing E. coli/ S. paratyphi/ V. parahaemolyticus in separate bottles. All the test solutions were incubated at 20o and also at 30oC in winter as well as at a certain depth.
Treated Autoclaved Estuarine Water: This test solution was used to evaluate whether cycloheximide has any inhibitory effect on the test organisms and also to study the survival in the absence of all biological factors.
Enumeration Techniques: The samples (1ml) from the test solution were taken and assayed after 3, 5, and 7 days using spread plate technique. The enumeration of bacteria was done after appropriate dilution using two plating media in parallel, one selective and the other non-selective. Selective and non-selective media were used in order to find out the injury exerted by the test solutions. The characteristic feature of the injured cells is that they fail to develop on the selective medium while maintaining the ability to grow on a non-selective medium. TSA was employed as non-selective medium while Eosin Methylene Blue (EMB) agar was used as selective medium for E. coli, Xylose Lysine Deoxycholate (XLD) agar for S. paratyphi and Thiosulfate Citrate Bile Salt Sucrose (TCBS) agar for V. parahaemolyticus as previously described by Abhirosh and Hatha (2005).
Statistical Analysis: The difference in the survival of the test organisms in cycloheximide treated, non-treated and autochthonous bacterial spp. added microcosms were analyzed using two way analysis of variance (ANOVA).
Results
The composition of dominant competing autochthonous bacterial genera encountered in the estuarine water is given in Table 1. Alcaligenes, members of the family Enterobacteriaceae, Aeromonas, Pseudomonas, Moraxella, Bacillus, Micrococcus and Actinomycetes were found to be the predominant genera in the estuarine water.
Table 1: Composition of the autochthonous bacteria in estuarine water.
| Gram-negative genera | Gram-positive genera |
| Alcaligenes | Bacillus |
| Enterobacteriaceae | Micrococcus |
| Aeromonas | Actinomycetes |
| Pseudomonas | |
| Moraxella |
Protozoans Encountered in the Estuarine Water: The protozoans found in the estuarine water samples are Coleps hirutus, Amoeba radiosa, A. proteus, Chylomonas, Arcella spp., Difflugia pyriformis, D. lobostoma, Vorticella campanula, Lionotus spp., Phacus pleuronectes, Oxytricha spp., Trachelomonas, Actinophrys sol, Paramoecium spp., Stylonichia spp., Euplotes spp. and Euglena spp. Among these, ciliates and flagellates exhibited greater diversity and abundance.
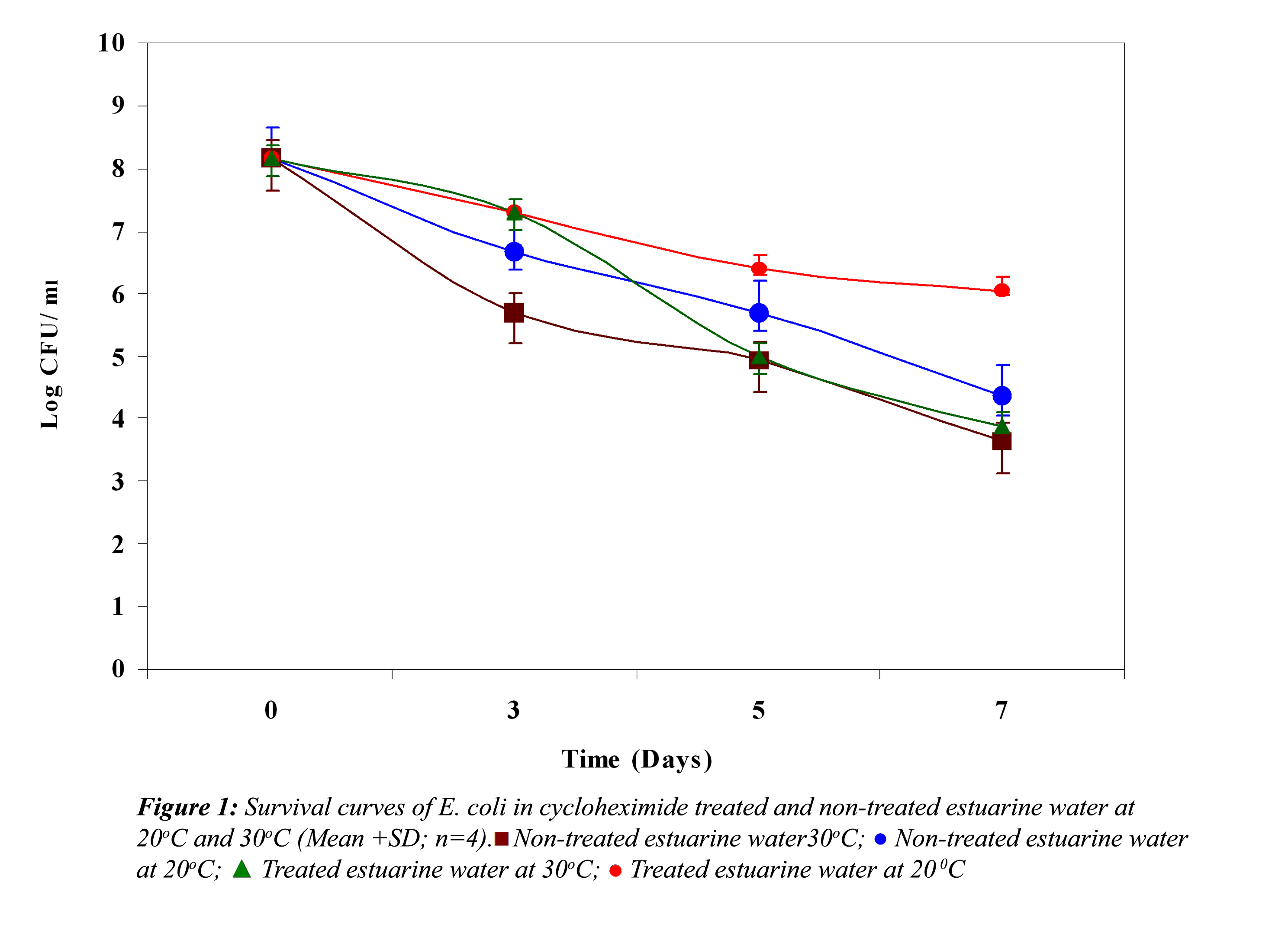
Effect of Biological Factors: Survival curves of E. coli, S. paratyphi and V. parahaemolyticus in non-treated and treated estuarine water at 20oC and 30oC are represented in Figure 1, 2 and 3, respectively. In non-treated estuarine water (Fig. 1), E. coli reduced nearly 5 logs at 30oC and almost 4 logs at 20oC after 7 days of exposure. E. coli showed better survival at 20oC than at 30oC. In cycloheximide-treated estuarine water E. coli declined almost 4 logs at 30oC whereas at 20oC it reduced only 2 logs indicating its high survival at 20oC.
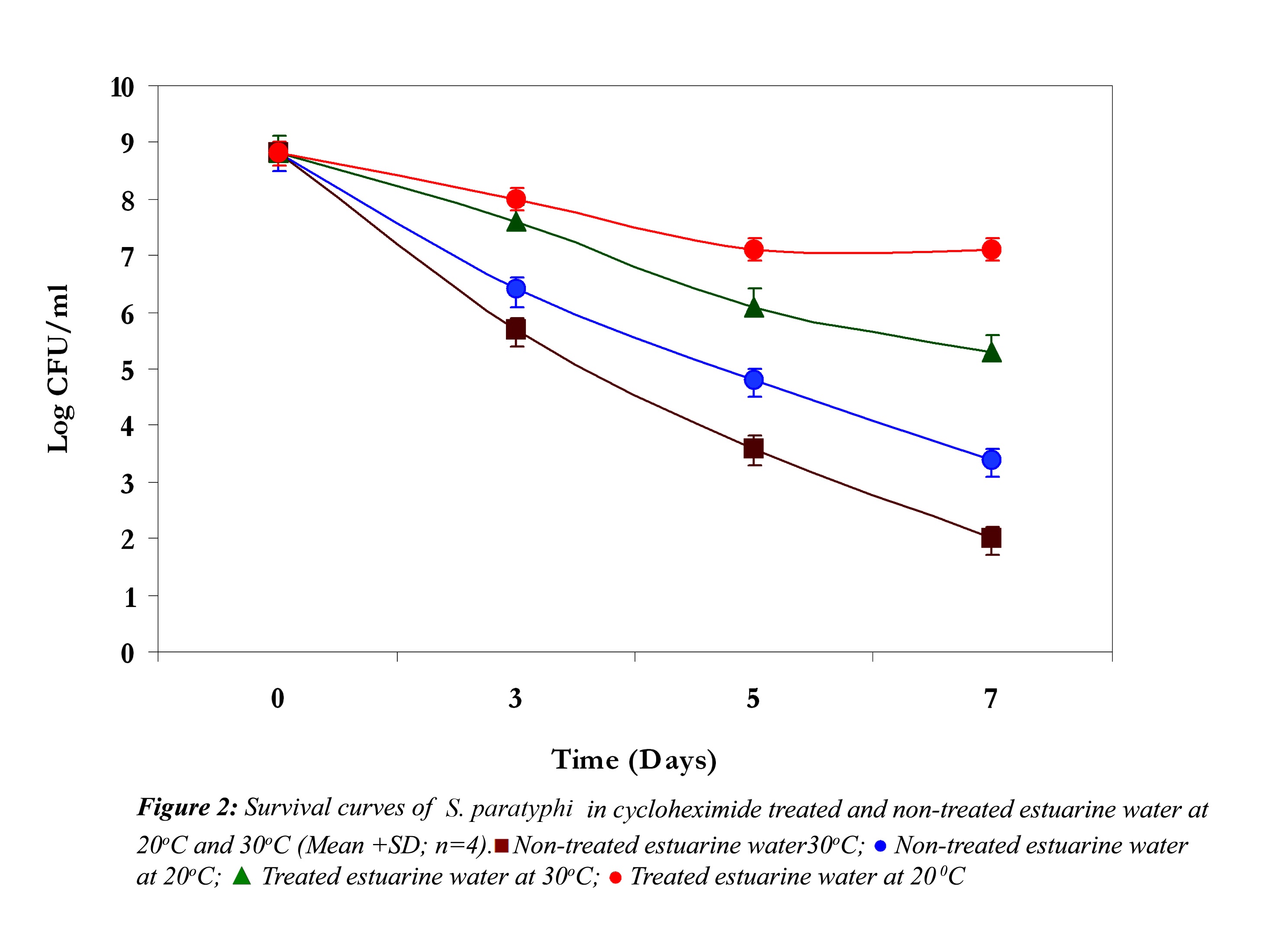
In non-treated estuarine water S. paratyphi demonstrated high die-off at 30oC and 20oC (Fig. 2). It showed a continuous steep reduction throughout the exposure time in estuarine water. The initial inoculum density was around 109 cfu /ml and it declined almost 7 logs at 30oC and nearly 6 logs at 20oC. However S. paratyphi exhibited improved survival at 20oC in cycloheximide treated and non-treated estuarine water. In cycloheximide treated estuarine water at 30oC it showed almost 3 log reduction in 7 days, but at 20oC it exhibited some kind of acclimatization towards the end of the experiment.
In non-treated estuarine water V. parahaemolyticus showed almost 4 log reduction at 30oC at the end of the fifth day (Fig. 3).
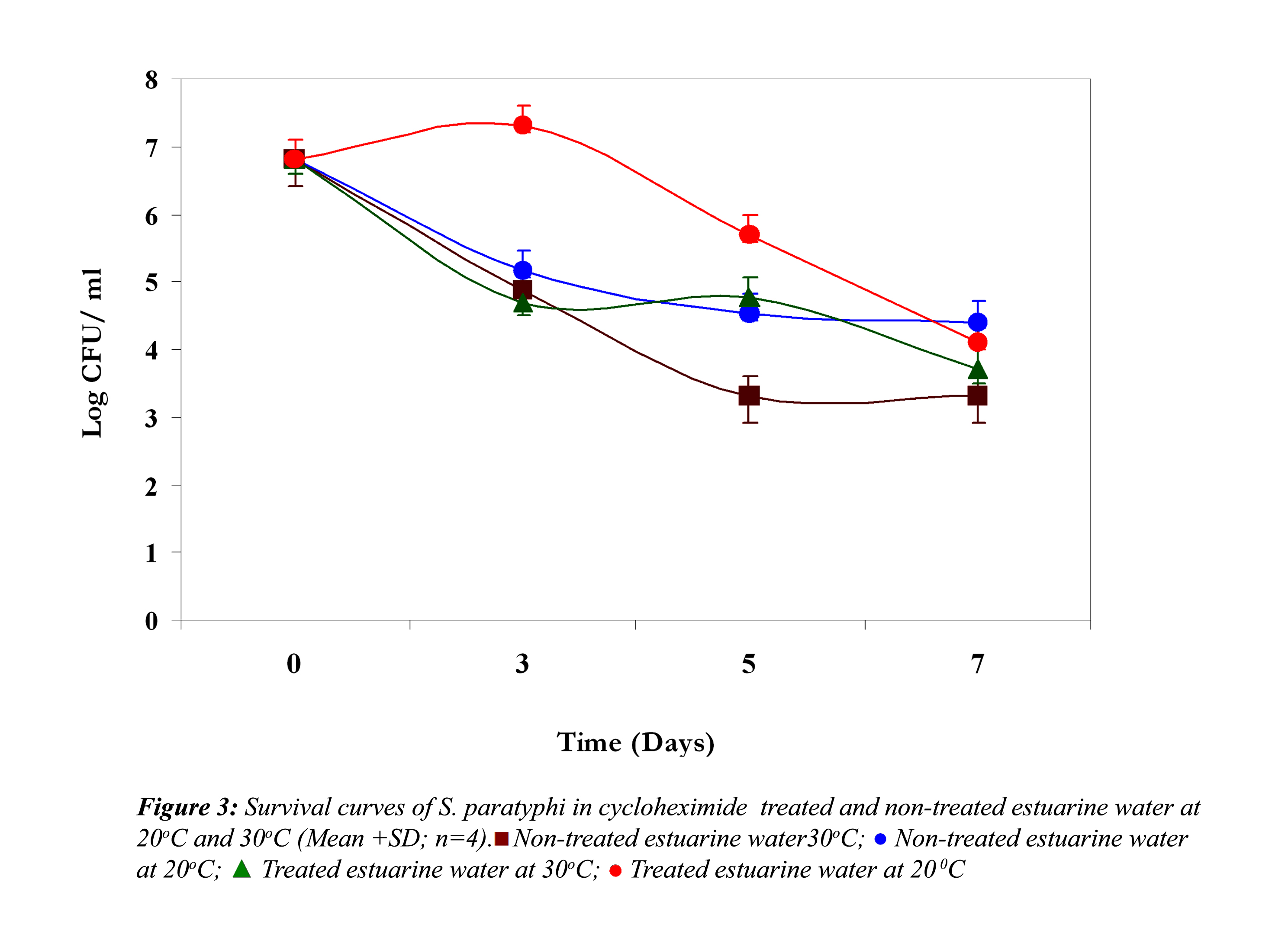
Thereafter it demonstrated a steady growth until the end of the experiment, but at 20oC it reduced only 2 logs, indicating their better survival at 20oC. However, in cycloheximide-treated water at 30oC, it demonstrated an initial reduction until the third day, and after that a slight growth, and then it again showed a reduction. On the other hand, at 20oC an initial growth was noticed; after that it showed a steep reduction till the end of the experiment.
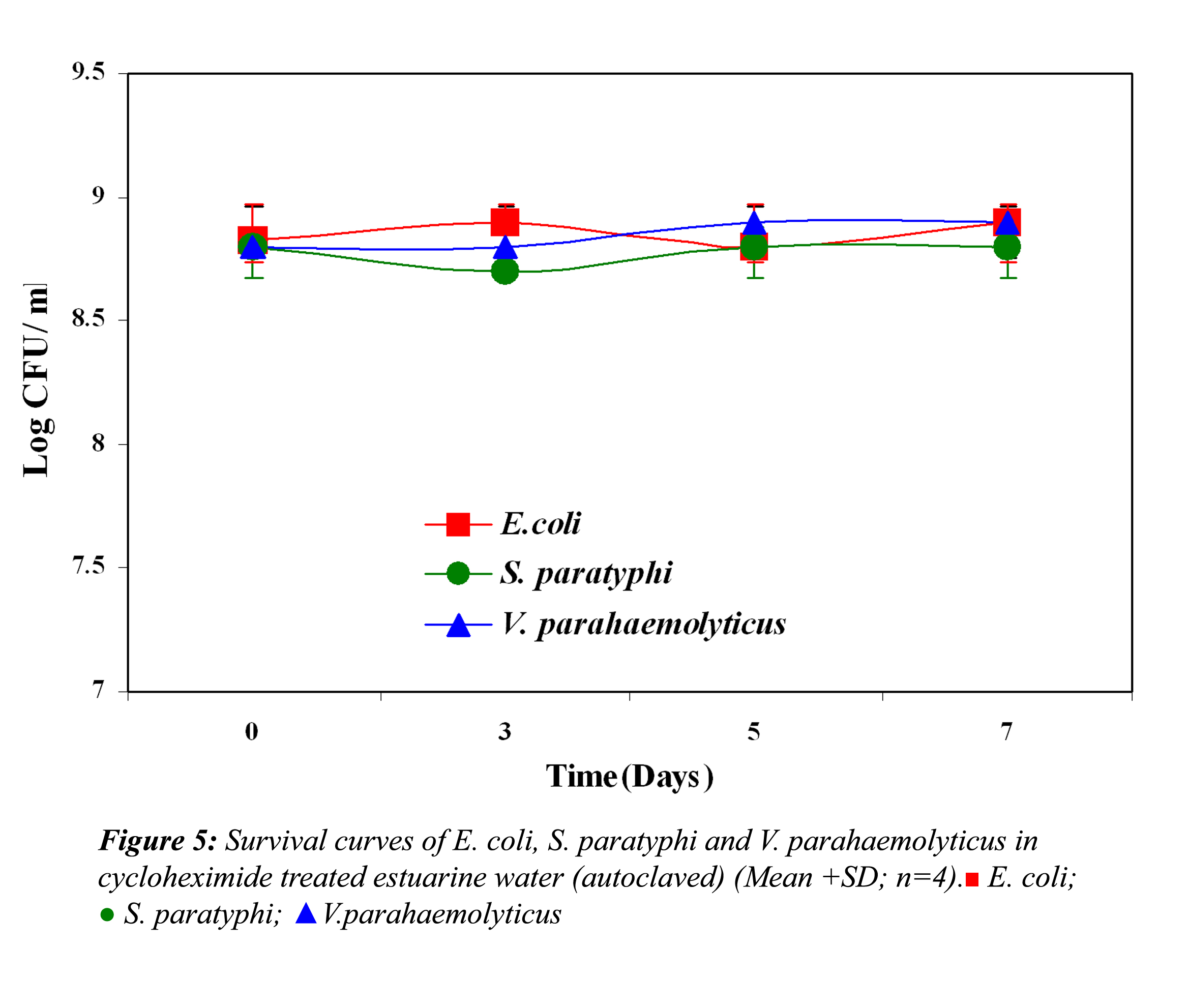
In all the above three cases the reduction in the cell density of E. coli, S. paratyphi and V. parahaemolyticus in cycloheximide-treated estuarine water may be considered as bacteriophage induced mortality. However no significant variation was observed in the survival of E. coli, S. paratyphi and V. parahaemolyticus in cycloheximide-treated and non- treated samples at both the temperatures (P>0.05). In autoclaved estuarine water the reduction of the test organisms was negligible (Fig. 5).
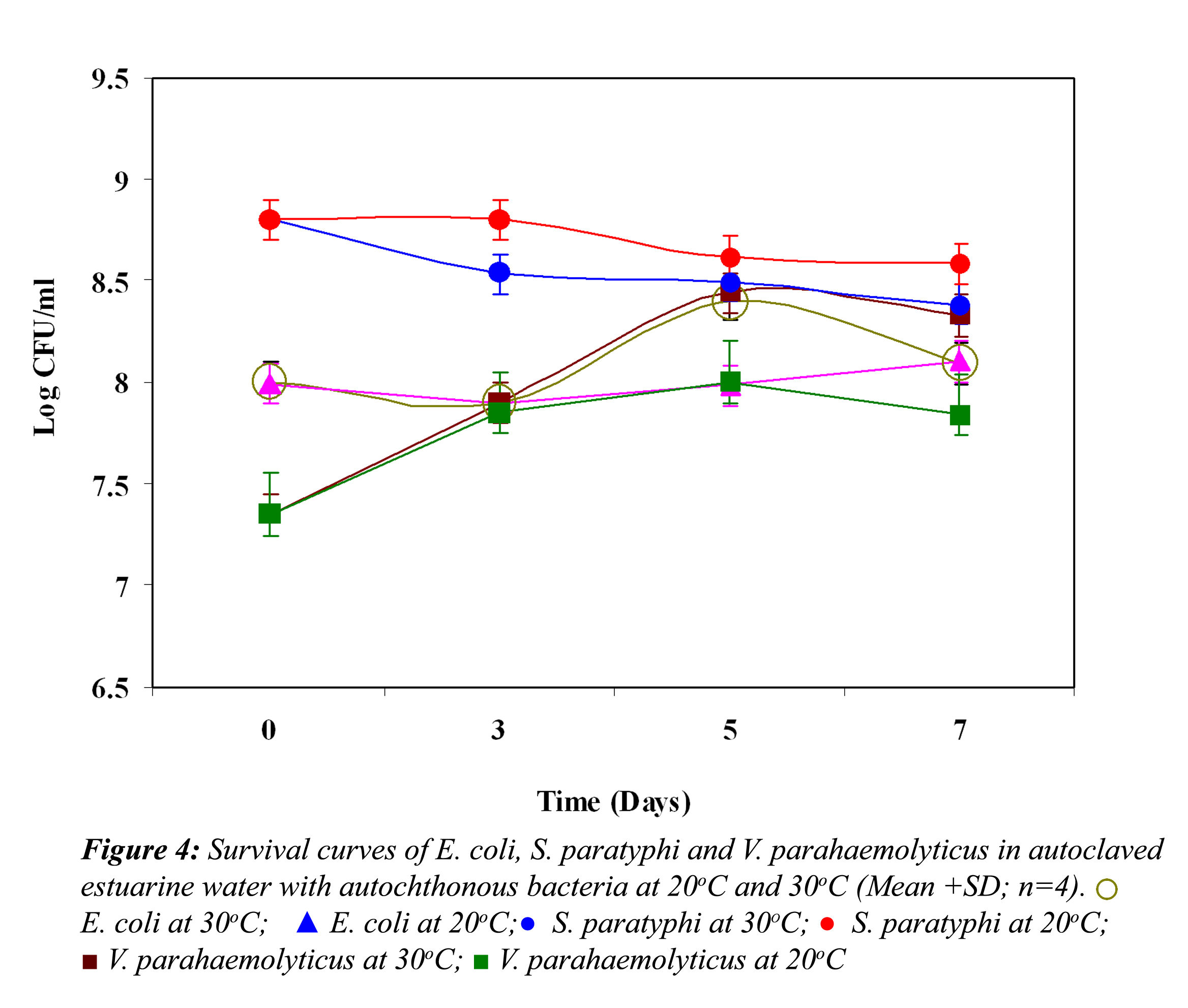
Effect of Autochthonous Bacteria: Represented in Fig. 4 are the survival curves of E. coli, S. paratyphi and V. parahaemolyticus in autoclaved estuarine water inoculated together with autochthonous bacteria isolated from estuarine water at different temperatures. E. coli showed slight reduction initially at both temperatures and thereafter it exhibited little growth and continued to decline toward the end of the experiment at 30oC, whereas it showed a slight growth at the end of the experiment at 20oC. S. paratyhi demonstrated a slight reduction during the initial stage of the experiment at 30oC, whereas at 20oC it showed an initial growth. The survival of S. paratyphi was slightly higher at 20oC than at 30oC. After that the numbers of cells did not change much. V. paraheamolyticus demonstrated a steady growth till fifth day of the experiment, and after that started to decline gradually at both the temperatures. No significant difference (P>0.05) was observed on the survival of E. coli, S. paratyphi and V. parahaemolyticus by the addition of autochthonous bacteria at both temperatures.
Discussion
When they were introduced into the non-treated estuarine water incubated at 20oC and 30oC, the mortality rate of E. coli, S. paratyhi and V. parahaemolyticus was high compared with cycloheximide-treated estuarine water. This indicates the effective role of protozoan predation on the survival of the test microorganisms. It has been well documented that protozoan grazing was the significant factor responsible for the removal of bacterial population in aquatic environments (Hahn and Hofle, 2001).
The observations of the present study are in agreement with the findings of the above finding that the destruction of E. coli, S. paratyhi and V. parahaemolyticus was very high in raw estuarine water (in the presence of protozoan predators) compared to cycloheximide-treated and autoclaved estuarine water where the predators (protozoan predators) were absent. The results support our previous observation in Cochin estuary that the biological factor—such as protozoan predation and bacteriophage—exerts high inactivation on E. coli and Salmonella typhimurium in estuarine water followed by sunlight (Abhirosh and Hatha, 2005).
The present study identified 19 protozoan species from the estuarine water. The most predominant groups were ciliates and flagellates. Hence the reduction of the test organisms in non-treated estuarine water was explained by the grazing effect of these flagellates and ciliates. In agreement with our results, Kalinowska (2004) found ciliates were the dominant taxa in different lakes, followed by heterotrophic flagellates. Kalinowska suggested that the density of bacteria was determined by ciliates. The results are also in agreement with the observation made by Hahn and Hofle (1998) who reported that Vibrio strain CB5 showed a 33% decline after inoculation with the flagellates. Simek et al. (1990) demonstrated that ciliates were important consumers of bacteria in aquatic environments. According to Simek et al. (1995) the grazing rate of a single individual of Vorticella was 4200 bacteria per hour, which also strengthened our observation since we recorded the occurrence of Vorticella in the estuarine water. However, in non- treated estuarine water, E. coli, S. paratyphi and V. parahaemolyticus survived better at 20oC than at 30oC. This is in agreement with the observation of McFeters and Stuart (1972) who reported that grazing pressure of microflagellates at lower temperatures is less effective, which may be a reason for the longer survival at 20oC.
The test organisms inoculated into test solution (containing all autochthonous bacteria) revealed that the competing autochthonous bacterial species had no effect on the test organisms. The enhanced survival of these organisms may be due to compounds produced by the bacterial population or by cryptic growth (when cells die, they generally produce compounds such as energy nutrient substrates through cell lysis). These compounds serve as a nutrient source for viable cells in the same population (Ryan, 1959). In agreement with our observation, Anderson et al. (1983) and Wcislo and Chrost (2000) found that in the presence of authochthnous bacteria, E. coli showed longer survival, which suggests some kind of stimulation (proto-cooperation) relationship which may enhance the survival of the test organisms. In contrast with our results, the role of competing autochthonous microflora on the removal of enteric microorganism has also been reported (McCambridge and McMeekin, 1981). The statistical results also revealed that the difference in the survival was not significant. Test microorganisms showed no or very little reduction in autoclaved estuarine water (control), which is devoid of any life forms. Similarly the direct effect of cycloheximide on the test microorganisms in autoclaved estuarine water was negligible.
Conclusions
In conclusion, the results indicated the effect of various biological factors on the survival of E. coli, S. paratyhi and V. parahaemolyticus in estuarine water. Among them, protozoan predation was the major biotic factor influencing the survival, followed by bacteriophages. However, autochthonous bacteria had little effect on the test organisms. The present study clearly indicated the self-purifying capacity of the natural aquatic environment and the role of different biological factors. In the event of pollution, if the addition of waste input exceeds the self-purifying capacity of the system, it may cause ecological imbalance and result in gross contamination. As we have seen in the estuarine water (autoclaved) without any biological factors, the survival of these pathogenic organisms was high. Therefore, the information would be important to understand the self-purifying factors and their relative importance in aquatic environment.
Acknowledgements
The financial support given to Abhirosh Chandran as Senior Research Fellowship (SRF) by the Council of Scientific and Industrial Research (CSIR), Government of India and Rajiv Gandhi National Fellowship by University Grants Commission (UGC), Government of India, is thankfully acknowledged. We are grateful to Mr. Sylesh Chandran, Junior Research Fellow, School of Environmental Science, Mahatma Gandhi University, for converting the figures into JPG format.
References
Abhirosh C, Hatha AAM (2005). Relative survival of Escherichia coli and Salmonella typhimarium in a tropical estuary. Wat Res 39:1397-1403.
Abhirosh C, Hatha AAM, Sherin V (2008). Increased prevalence of indicator and pathogenic bacteria in Vembanadu Lake: A function of salt water regulator, along south west coast of India. J Wat Hlth 06: 539-546.
Alonso MC, Rodriguez V, Rodriguez J, Borrego JJ (2000). Role of ciliate, flagellates and bacteriophages on the mortality of marine bacteria and on dissolved DNA concentration in laboratory experimental systems. J Exp Mar Bio Eco 244: 239-252.
Anderson JC, Rhodes MW, Kator HI (1983). Seasonal variation in the survival of E. coli exposed in situ in membrane diffusion chambers containing filtered and nonfiltered estuarine water. Appl Environ Microbiol 45: 1877-1883.
Batish SK (1992). Fresh water Zooplankton in India. Oxford and IBH Publishing Co. Pvt. Ltd. New Delhi: 17-60.
Buchanan RE, Gibbons NE (1984). Bergeys manual of determinative bacteriology, 8th ed. Williams and Wilkins, Baltmore, MD: 1268.
Catalao DLP, Rheinheimerà G, Borrego JJ (2000). Microbiological Pollution of Ria Formosa (South of Portugal). Mar Poll Bull 40:186-193.
Davies CM, Long JAH, Donald M, Ashbolt NJ (1995). Survival of faecal microorganisms in marine and freshwater sediments. Appl Environ Microbiol 61:1888-1896.
Duhamel S, Domaizon-Pialat I, Personnic S, Jacquet S (2006). Assessing the microbial community dynamics and the role of bacteriophages in bacterial mortality in lake Geneva. Revue des Sciences de l’Eau 19: 115-126.
Fuhrman JA, Noble RT (1995). Viruses and protists cause similar bacterial mortality in coastal seawater. Limnol Oceanogr 40: 1236-1242.
Hahn MW, Hofle MG (2001). Grazing of protozoa and its effect on populations of aquatic bacteria. Microb Ecol 35: 113–121.
Hahn MW, Hofle MG (1998). Grazing pressure by a bacteriovorous flagellate reverses the relative abundance of Comamonas acidovorans PX 54 and Vibrio strain CB5 in chemostat cocultures. Appl Environ Microbiol 64:1910-1918.
Hatha AAM, Abhirosh C, Mujeeb Rahiman KM (2004). Prevalence of diarrhegenic serotypes of Escherichia coli in the Cochin estuary, along west coast of India. Indian J Mar Sci 33: 238–242.
Hennes KP, Simon M (1995). Significance of bacteriophages for controlling bacterioplankton growth in a mesotrophic lake. Appl Environ Microb 61: 333-340.
Jacquet S, Domaizon I, Personnic S, Ram ASP, Hedal M, Duhamel S, Sime-Ngando T (2005). Estimates of protozoan- and viral-mediated mortality of bacterioplankton in Lake Bourget (France). Freshwat Biol 50:627–645.
Kalinowska K (2004). Bacteria, nanoflagellates and ciliates as components of the microbial loop in three lakes of different trophic status. Pol J Ecol 52: 19–34.
Kaper JB, Nataro JP, Mobley HLT (2004). Pathogenic Escherichia coli. Nat Rev 2: 123–140.
Kindhauser MK (2003). Global defence against the infectious disease threat. Communicable Diseases 2002. World Health Organization, Geneva.
Le Guyader F, Pommepuy M, Cormier M (1991). Implantation of Escherichia coli in pilot experiments and the influence of competition on the flora. Can J Microbiol 37: 116-121.
Marano NN, Daniels NA, Easton AN, McShan A, Ray B, Wells JG, Griffin PM, Angulo FJ (2000). A survey of stool culturing practices for Vibrio species at clinical laboratories in Gulf Coast states. J Clin Microbiol 38: 2267-2270.
McCambridge J, McMeekin TA (1981). Effect of solar radiation and predacious microorganisms on faecal and other bacteria. Appl Environ Microbiol 41: 1083-1087.
McFeters GA, Stuart DG (1972). Survival of coliform bacteria in natural field and laboratory studies with membrane filters chambers. Appl Microbiol 24: 805-811.
Ryan FJ (1959). Bacterial mutation in a stationary phase and the question of cell turnover. J Gen Microbiol 21: 530–549.
Sherr EB, Sherr BF (2002). Phagotrophy in aquatic microbial food webs. In: Newell S (eds) Manual of Environmental Microbiology. ASM Press, Washington DC: 409–418.
Simek K, Bobkova J, Macek M, Nedoma J, Psenner R (1995). Ciliate grazing on picoplankton in a eutrophic reservoir during the summer phytoplankton maximum: A study at the species and community level. Limnol Oceanogr 40:1077–1090.
Simek K, Macek M, Seda J, Vyhnalek V (1990). Possible food chain relationships between bacterioplankton, protozoans, and cladocerans in a reservoir. Int Rev ges Hydrobiol 75:583–596.
USEPA (1978). Microbiological methods for monitoring the environment, water and wastewater. US Environmental Protection Agency, Cincinnati, Ohio.
Wcisło R, Chróst RJ (2000). Survival of Escherichia coli in freshwater. Pol J Environ Stud 9:215-222.
Weinbauer MG, Hofle MG (1998). Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl Environ Microbiol 64: 431–43.
Discussion with Reviewers
Helvi Heinonen-Tanski1: A part of your work has been paid by the local people as tax payers. How do you think that your work is giving benefit for them?
Abhirosh Chandran: This is indeed a very relevant question. As you said, part of the funding is from the tax payers money and it should have some relevance and benefit to these people. Kumarakom Estuary is a life-line for people around this region since it supports the livelihood of fisherfolk. Water from the estuary is mainly used for household cleaning and occasionally for drinking. Boats use the estuary to move between islands. Considering the importance of this estuary to the daily life of people in this region, our primary objective was to look at the microbial pollution in the estuary and give some primary data regarding the risk associated with the usage of this water in a careless manner. At the same time we also wanted to evaluate the self purification potential of this water body as a function of the self-contained factors such as the biotic factors, which include autochthonous microflora, protozoans and bacteriophages. Our results indicated that the system has got considerable self-purifying capacity, provided the waste input into the system is managed within the levels that could be handled by the Kumarakom estuary. This observation is very significant as we can interact with the local people and inform them about the need to be more responsible for the long-term benefit of their own. The results are also passed on to the local authorities so that they can implement measures to control point source effluent inputs and irresponsible dumping of waste into the system. These measures could ultimately benefit local people.
Heinonen-Tanski: In Finland we have very few cases of salmonellosis in human or animal populations. But we and many other organizations make many salmonella analyses. The result is usually that nothing is found (= less than the detection limit). We claim that one euro in salmonella analyses saves nine euros in health costs since the number of salmonellosis patients in hospitals is really low (almost all cases in people who have been working or traveling in foreign countries). Could you estimate what should be done in order to reduce water-borne diseases in Kerala (assuming that Kerala is the most developed state in India)?
Abhirosh: As you said, Kerala is the most developed state in India. However, like many other states in India, Kerala is also far behind in solid and liquid waste-treatment facilities with subsequent contamination of most surface-water resources. Though Kerala is the most literate state in India, the worrying aspect is the socially irresponsible behavior of large section of Keralites. However, they take many measures at home to have safe water and food. Socially irresponsible behavior, such as careless disposal of wastes into nearby streets or water bodies, is causing serious environmental degradation. This degradation ultimately comes back to the people by way of many diseases. Studies like the ones carried out by us reveal the problem to a wider public audience in a very scientific manner. As of now, the Kerala government is quite aware of the problem. The government is implementing multi-pronged strategies including strict enforcement of antipollution laws. Implementation of a proper treatment facility for liquid and solid waste would provide pure drinking water and definitely reduce water-borne diseases in Kerala to a greater extent. To attain this goal of public participation is paramount. The effective and appropriate action plan from public health personnel and the government is necessary in order to safeguard the public health. I believe that the health of the people is the wealth of the Nation.
Heinonen-Tanski: For salmonella you made direct determination method. Could you see any benefit to make a biphasic pre-enrichment + enrichment analysis?
Abhirosh: For the enumeration of Salmonella from the lake water we have used the biphasic pre-enrichment + enrichment analysis. But for the survival experiment we have used only the direct determination method because we have inoculated about 109 Salmonella cells in the microcosm and used selective media for the enumeration. The recovery was very good. I think it may not be necessary to use biphasic pre-enrichment + enrichment analysis to determine Salmonella from already inoculated microcosm. But it is necessary when it is determined from a lake water sample because the recovery may be very low. So I think that direct determination and biphasic pre-enrichment + enrichment analysis may not cause much difference in survival studies.
1 Adjunct Professor, Department of Environment Science, University of Kuopio, Finland
1 Correspondence: heinotan@uku.fi
