 Sulfate’s Critical Role for Maintaining Exclusion Zone Water: Dietary Factors Leading to Deficiencies
Sulfate’s Critical Role for Maintaining Exclusion Zone Water: Dietary Factors Leading to Deficiencies
Seneff S1*, Nigh G2
1Computer Science and Artificial Intelligence Laboratory, MIT Cambridge MA 02139, USA; Seneff@csail.mit.edu
2Naturopathic Oncology, Immersion Health, Portland, OR 97214, USA; drnigh@immersionhealthpdx.com
*Correspondence: Stephanie Seneff seneff@csail.mit.edu
Keywords: Structured water; sulfate; exclusion zone; cobalamin; eNOS; glyphosate; metformin; vegan diet
Submitted: June 21, 2019; Revised: September 30, 2019; Accepted: October 22, 2019; Published: December 18, 2019; Available Online: December 18, 2019
Abstract
Biological water exists in at least two distinct forms: bulk and interfacial. While the former possesses widely understood properties, little has been written about the formation, maintenance, and functional role of interfacial water in living systems. In this paper we equate interfacial water with Exclusion Zone (EZ) water described by Pollack and propose that it is the sulfate molecule that plays a fundamental role in providing the interfacial negative charge that builds and maintains the EZ in biological systems. We further propose novel roles for endothelial nitric oxide synthase (eNOS), erythrocytes, and cobalamin in sulfate production and ongoing regulation. Two exogenous agents, the diabetes drug metformin and the herbicide glyphosate, and one lifestyle factor, vegetarianism/veganism, can contribute to reduced sulfate production and subsequent loss of EZ water. A set of compensatory changes in the body, often normally considered to be discrete pathologies, serve to reestablish adequate sulfate supply in the face of these and other detrimental impacts on sulfate production. Finally, we review additional pathologies associated with cobalamin deficiency and suggest that they, too, can be linked to the restoration of sulfate metabolism.
Introduction
Water is essential for life, but the significance of water’s unique properties that make it essential is an under-represented topic in the research literature. A small group of dedicated researchers have devoted their lives to understanding the unique properties of water. Gerald Pollack PhD, and Professor at the University of Washington, is perhaps the best-known member of this group, due in part to his remarkable skill in translating dense science into terms that are comprehensible to the non-expert. He has succeeded in popularizing the notion of EZ water, which is a “fourth phase” of water (beyond solid, liquid, and gas) characterized by a regularized crystalline hexamer structure, yielding a gel that excludes solutes and also extrudes protons (Pollack and Clegg, 2008). As a result, the gel itself is negatively charged, and a voltage drop is created at the interface with neighboring “unstructured,” or bulk, liquid water. This voltage drop can be as high as -200 mV between EZ water and the adjacent unstructured water. The charge separation that is induced forms a battery that can supply energy to the tissues (Hwang et al. 2018). This gel phase of water is the dominant phase present in the human body.
Highly hydrophilic synthetic materials such as Nafion and ceramic powder can be used to simulate biological membranes. Water exposed to these materials separate into a heterogeneous structures with sharp boundaries dividing EZ water from unstructured water. The EZ water has a negative electric potential, whereas ordinary low-density water has a positive polarity. In an experimental study of water exposed to ceramic powder at a 3% concentration, the zeta potential (ZP) was measured to be -37.7 mV, and the voltage drop reached a maximum at -80 mV (Hwang et al. 2018). This is a model for the arrangement of water molecules in the vasculature, where hydrophilic substances bound to the endothelial membrane create EZ water that interfaces with unstructured water in the blood, and this creates a battery that supplies energy to the endothelial cells. A similar process takes place surrounding all the cells in the tissues. The charge separation and battery effect are an important aspect of water in the support of life, and we will be reviewing the evidence behind these assertions.
Evidence for the Unique Structuring of Water Within Living Systems
The recognition of water as holding a unique position in the processes of life dates back hundreds of years, at least to the 16th century, when Paracelsus proclaimed that water is “the matrix of the world and of all its creatures.” (Jolande, 1988) With the advent of modern technologies, however, that has allowed for a much more detailed understanding of the role water is playing in the maintenance of the living state and its relationship to health and disease. We briefly review, here, some representative studies in a large body of literature.
Szent-Gyorgyi (1956) noted that the cytoplasm of our cells contains little or no “random water,” but instead contains water that is highly constrained, much like a “liquid ice.” The recognition of this unique structuring of water grew as new technologies allowed for closer investigations. In 1971, Damadian (1971) first published his findings, obtained using NMR, that it was evident that “cancerous tissue has a lower degree of organization and less water structure than normal tissue.” Later imaging techniques such as MRI and CT confirmed the same loss of water structure regarding tumors and other pathological tissue (Unger et al., 1988). Iwama et al. (1992) reported a significant difference in spin-lattice cross-relaxation times between control water protons vs. protons in water of peritumoral edema, noting that loss of water structure may be an early event in edema formation.
Vink and Duling (2000) noted a 0.4-0.5µm thick gelled layer of water lining the endothelium of all healthy vessels. This layer was noted to regulate solute penetration based upon molecular size and charge. The authors had coincidentally also referred to this layer as an “apparent endothelial exclusion zone.” Pizzitutti et al. (2007) discussed the “anomalous behavior” of hydration water surrounding proteins. Apparently unfamiliar with the research into biological EZ water, the authors observed that “Water around biomolecules slows down with respect to pure water,” and “[t]he origin of such behavior remains elusive.” It is highly likely that they were witnessing the altered properties of water forming the EZ layer around the proteins under investigation.
Studying collagen, Melacini et al. (2000) noted the importance of water in stabilizing its triple helix crystalline structure. They found that water within the collagen helix forms a “semi-clathrate-like structure that surrounds and interconnects triple helices in the crystal lattice.” Water within the matrix of bone is likewise highly structured, a structuring that has been found associated not only with the organic macromolecules such as collagen and proteoglycans, but with the mineral surfaces as well. Water, in fact, seems to play a foundational role in orienting mineral nanoparticles into parallel arrangements within the bone matrix, providing this orientation even in the absence of organic molecules (Duer and Veis, 2013).
An excellent 2007 review by Le Bihan, (2007) catalogues the wide-ranging evidence to date that biological water has both unique structure(s) and physical properties. The following year, Ball (2008) expanded that review to cover the active role water plays in a wide range of cellular and physiological functions. Most critically, it has become evident that most biological water, both intra- and extracellular, is highly structured and does not exist in the bulk state. Pathology, in turn, is associated with the loss of water structure. Most recently Sharma et al. (2018) found that several nutrients and medications had the effect of extending (thickening) the EZ layer in an in vitro model, while the herbicide glyphosate (the active ingredient in Roundup®) was found to narrow the EZ layer. The authors note the documented role of the EZ in stabilizing protein folding, and propose that the positive or negative health impact of various nutrients or toxicants may be due to their effect on biological EZ water.
Maintenance of EZ water is important both intracellularly and extracellularly. A review by Kerch (2018) differentiates between the “tightly bound water” associated with healthy cells and tissue in a wide range of conditions, and the “loosely bound water” associated with an equally wide range of diseases and with aging.
EZ Water and Sulfate
Given this critical role of EZ water for maintenance of normal cellular physiology and organismal health, we turn now to the specific ways in which sulfate, associated with various macromolecules, contributes to this water structuring and to general homeostasis of the organism. We show that sulfate plays a role in maintaining the EZ water lining blood vessels and in maintaining the proper viscosity of the circulating blood.
Glycosaminoglycans
As described in more detail below, we hypothesize that water is maintained in this gel form through the special assistance of sulfate molecules that are anchored to the extracellular matrix of most cells in the body. Thus, sulfate takes on a dual role: as a vital structural component of the matrix, and as the dominant water-structuring component within that matrix. The sulfate molecules are complexed with long amino-sugar chains to form various biologically interesting molecules such as heparan sulfate, chondroitin sulfate, keratan sulfate and dermatan sulfate. These chains, collectively, are called glycosaminoglycans (GAGs), and are either attached to membrane-bound proteins or attached to free-floating proteins bound to hyaluronan, a very large polysaccharide that sprawls across the extracellular matrix space and also helps to maintain the gel (Seneff et al., 2015). These sulfated amino-sugar chains are also attached to lipid molecules, such as ceramide, to form the sulfonated lipid, sulfatide (Takahashi and Suzuki, 2012).
Chaotropes and Kosmotropes
Contrary to most of the extracellular tissue water, the water in the circulating blood must remain in the liquid phase. Small ions can be characterized by their effects on water structuring/destructuring along a scale called the Hofmeister scale, where ions that tend to structure water (i.e., form a gel) are called “kosmotropes,” representing one extreme, and ions that destructure water (maintaining a liquid phase) are called “chaotropes.” (Russo, 2008) Sulfate is among the strongest of the biological kosmotropes, and this is what makes it so important in maintaining the gelled water in the extracellular matrix of most cells, where the relative sulfate concentration is much higher than in the blood. Its presence in the bloodstream will also have a gelling effect, thereby increasing blood viscosity.
By contrast, nitrate is a chaotrope, and it will therefore act to decrease blood viscosity and maintain adequate flow. Both free nitrate and free sulfate are present in substantial amounts in the blood, and are highly regulated and maintained in a constant balance. One effect of this tight regulation is to keep the blood viscosity within a narrow range, the high shear rate measurement spanning between 14.28 and 19.40 (208 Sec-1) from critically low to critically high, respectively (Nwose, 2010). Free sulfate levels in the blood are maintained usually at the level of 0.3 to 0.4 micromolar (Morris and Levy, 1983).
Endothelial Nitric Oxide Synthase
Endothelial nitric oxide synthase (eNOS) is one of three isoforms of an enzyme that synthesizes nitric oxide (NO) from arginine, the other two being neuronal NOS (nNOS) and inducible NOS (iNOS) (Knowles and Moncada, 1994, Griffith and Stuehr, 1995). The isoform, eNOS, is highly expressed in endothelial cells lining the vascular wall; hence its name. The enzyme has a very complex requirement for cofactors, and a very complex regulatory mechanism (Zweier et al., 2011, Zou et al., 2002). The cofactors include heme, tetrahydrobiopterin (BH4), nicotinamide adenine dinucleotide phosphate (NADPH) and the nucleotides flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN). It also houses a zinc atom in a cavity formed between two molecules of the eNOS dimer. eNOS can be found bound to caveolin in caveolae formed in the membrane of the endothelial cell, and this is considered to be an inactive state. Calcium entry and subsequent calmodulin binding and phosphorylation cause eNOS to leave the membrane, and become activated to produce NO (Takahashi and Mendelsohn, 2003).
Much has been written about a dysfunctional “uncoupled” state of eNOS where it produces superoxide rather than NO (Zweier et al., 2011, Duan and Kwan, 2011, Landmesser et al., 2003). This is very dangerous because the superoxide reacts with NO to form peroxynitrite (ONOO−) a highly damaging oxidizing agent. It has been proposed that eNOS is a moonlighting enzyme (Seneff et al., 2015, Seneff et al., 2012), that synthesizes not only NO but also sulfur dioxide (SO2), and that it switches between these two under exquisite external control, mediated by the viscosity of the flowing blood (Melkumyants et al., 1989) and communicated through electromagnetic signaling. This is in fact the reason for its complex control mechanisms. We believe evidence for this dual role of eNOS is compelling, and that it is further supported by evidence we present here.
In this model, eNOS synthesis of SO2 is catalyzed by sunlight. Infrared light expands the EZ water (Chai et al., 2009), UV light mobilizes electrons within the EZ water, and flavins bound to eNOS respond to blue light by emitting electrons needed to oxidize the sulfur atom (Seneff et al., 2015, Seneff et al, 2012). Light, including infrared, provides the energy source for the formation of EZ water (Hwang et al. 2018).
While hypothetical, this model not only explains several anomalies associated with eNOS within red blood cells (RBCs), it also accounts for the tight feedback regulation between these two cardinal viscosity regulators, SO4-2 and NO3–. A consequence is that eNOS dysfunction, caused by environmental toxins and resulting in the release of superoxide, leads not only to local damage, particularly in the endothelial wall, but also to impairment of the SO4-2 / NO3– balance.
EVSP, NO and Viscosity Regulation
Flowing blood induces an electromagnetic field called the electrokinetic vascular streaming potential (EVSP), which acts as a signal to stimulate endothelial cells to release NO (Trivedi et al., 2013). This field can increase the response of the endothelial wall to ATP stimulation by up to a factor of seven. The magnitude of the signal depends on the zeta potential (ZP), which in turn reflects the amount of negative charge carried on the surface of the RBCs, establishing a hydration shell. There is evidence that EZ water extends beyond this hydration shell for several micrometers (Yoo et al., 2011).
The EVSP also carries a frequency component synchronized to the heartbeat. The response intensity (production of NO) rises with an increase in the heart rate. As previously stated, nitrate, an oxidation product of NO, is a chaotrope that destructures water, reducing the viscosity of the blood and improving flow rates. Increased flow is beneficial for rapid distribution of oxygen and nutrients to over-exercised muscle cells associated with intense exertion, which also sharply increases the heart rate.
The normal ZP of RBCs is -15.7 millivolts (Fernandes et al., 2011). The source of the negative charge in the RBC membrane has been little studied, but it is logical that sulfate is an important contributor, since it is a negatively charged molecule and a prominent component of the cell glycocalyx. Erythrocyte membranes contain both sialic acid (Syed and Rizvi, 2013) and sulfated GAGs (Srikanth et al., 2012), and these are probably covalently linked, forming complex sugar structures similar to those described in Roux et al., (1988). Srikanth et al. (2012) demonstrated that RBCs contain both chondroitin sulfate and dermatan sulfate, two of the sulfated GAGs that populate the extracellular matrix of most other cells. In this study, most of the disaccharides that were present in the RBC extracellular GAGs were sulfated. Sulfate is estimated to contribute around 17% of the negative charge in typical sulfated oligosaccharides (Roux et al., 1988). We predict that it contributes a much larger percentage in RBCs, because the GAGs in RBCs are highly sulfated.
A study comparing diabetic patients to normal controls revealed that the RBCs of diabetics had reduced levels of sialic acid, which was associated with increased risk of aggregation (rouleaux formation) and adherence to the vascular endothelial wall. The authors proposed that reduced negative charge on the RBC membrane led to these defects (Rogers et al., 1992). It has been demonstrated that RBCs lose their negative charge and their sialic acid as they age, and this may be an important marker of senescence (Syed and Rizvi, 2013).
eNOS in RBCs
Red blood cells express eNOS in relatively high concentrations, and this has presented a puzzle to researchers, because nitric oxide would be expected to interfere with hemoglobin’s ability to carry oxygen, like carbon monoxide does (Mehta et al., 2000). Furthermore, RBCs have poor support for transport of the substrate arginine across their membranes. They produce high levels of arginase, which swiftly clears any arginine that enters the cell, thus apparently rendering their eNOS useless (Kang et al., 2000). In addition, the eNOS of RBCs is mostly concentrated near the plasma membrane (Kleinbongard et al., 2006). In other cells, as mentioned previously, membrane-bound eNOS is considered inactive with respect to NO (Chen et al., 2012).
A considerably more plausible model, as we have suggested, is that RBCs use their membrane-bound eNOS to synthesize sulfur dioxide (SO2) rather than NO (Seneff et al., 2015, Seneff et al, 2012). Just as NO spontaneously oxidizes to nitrite and then to nitrate, SO2 would also be spontaneously oxidized to sulfite and then enzymatically oxidized to sulfate by sulfite oxidase. Keeping in mind that sulfate and nitrate are at opposite poles of the Hofmeister series, with sulfate being a kosmotrope and nitrate being a chaotrope, it could be intelligent design in biology to have a single enzyme switch between sulfate and nitrate synthesis under exquisite control of signals derived from blood parameters to maintain blood viscosity within narrow limits. This model places the RBC at the center of systemic blood viscosity homeostasis though regulation of EZ water in the circulatory system and beyond.
Cholesterol and Cholesterol Sulfate
Many sterols are sulfated in transit, and cholesterol sulfate (CS) is among the most common sulfated sterols present in the blood plasma (Strott and Higashi, 2003). Sulfation makes cholesterol amphiphilic, rendering it unnecessary to package it up inside lipid carrier molecules such as high density liporprotein (HDL) and low-density lipoprotein (LDL). CS is produced in large amounts in the skin and released into the membranes of HDL particles, which likely helps distribute it to the tissues. CS’s rate of inter-membrane exchange is approximately ten times faster than that for cholesterol, allowing it to more readily enter liposomal membranes (Rodriguez et al., 1995). The sulfated cholesterol molecules would provide a negatively charged field around the HDL particle, as well as creating an EZ water shell that can protect the particle from oxidation and glycation damage.
High serum cholesterol (e.g., high LDL particles) is a central pathogenic factor in endothelial dysfunction, causing a reduction in the release of NO, and this effect is likely mediated through upregulation of caveolin expression (Feron et al., 1999). The eNOS bound to caveolin at the plasma membrane is inactive with respect to NO synthesis. However, if membrane-bound eNOS can synthesize SO2 instead, then it becomes plausible that excess serum cholesterol would induce synthesis of SO2 to support sulfate synthesis and its conjugation with cholesterol via a sulfoconjugase enzyme to form CS. This would be particularly important for membrane-bound eNOS in RBCs, because these cells normally carry significant amounts of CS in their membrane. This helps maintain their biconcave shape (Przybylska et al., 1998) and also contributes significantly to the maintenance of an extended hydration layer that is dependent upon CS content (Faure et al., 1996).
Within the three-dimensional compartment of the cytoplasm of RBCs, water is predominantly in the bulk state. Only 10% is interfacial, associated with the hydration layer around hemoglobin molecules (Stadler et al., 2008). At the two-dimensional RBC membrane, though, associated water is gelled, or, as we now understand it, EZ water. As noted by Schneider & Schneider in their seminal study of RBC membrane water content, the relatively high hydrations at higher humidities may reflect a gel-type swelling or capillarity of the membrane matrix (Schneider et al., 1972). This membrane-associated EZ water is dependent upon the CS content of the membrane.
Individuals with a genetic defect in the steroid sulfatase gene leading to ichthyosis have been found to have extremely elevated levels of CS in the membranes of their RBCs as well as in the plasma (Bergner and Shapiro, 1981). Their plasma CS levels were measured to be on average 3,300 micrograms/100 ml as compared to under 350 micrograms/100 ml in normal adults. Their RBCs were even more extreme, containing on average 7,500 micrograms/100ml compared to less than 300 micrograms/100 ml in normal adults. This represents a 30-fold increase in the membrane CS content.
Collectively, these findings suggest to us two important, previously unknown roles that RBCs normally play in physiology. First, through their sheer volume they contribute to the overall viscosity of the blood through this “entrapment” of local water into an EZ layer at the RBC surface. Second, they play a central role in the distribution of cholesterol to the tissues. In this regard, the removal of the sulfate ligand is an essential part of the process. We hypothesize that, under normal conditions, CS is only housed temporarily in the RBC membrane. Once released, it decomposes into cholesterol and sulfate, and these two nutrients are supplied to the endothelial cells lining the capillaries and to the tissues and organs. Sulfate anions can be attached to the extracellular GAGs to reinforce their sulfate supplies.
In this model, high serum cholesterol is potentially an indicator of cholesterol sulfate deficiency. Since sulfation renders the molecule water soluble, impaired sulfation necessitates that cholesterol be carried within a lipid particle in an esterified form. At the same time, the sulfate anion, secured in a lipid membrane and bound to cholesterol, is prevented from acting as a kosmotrope to gel the circulating blood and disrupt flow.
Garlic is universally recognized as a nutritionally important food that is protective against cardiovascular disease, atherosclerosis, hyperlipidemia, thrombosis, hypertension and diabetes (Banerjee and Maulik, 2002). It is believed that much of its benefit derives from the fact that it is an excellent source of sulfane sulfur (diallyl sulfide, diallyl disulfide and diallyl trisulfide) (Iciek et al. 2016). RBCs extract sulfur from garlic and release gas, which then induces vasorelaxation acting as a gasotransmitter (Benavides et al., 2007). Glucose processing through the pentose phosphate pathway in RBCs restores NADPH to the reduced state following its participation in redox reactions in the cell. We suggest here that among these redox reactions is NADPH’s involvement in the mobilization of electrons to catalyze the synthesis of SO2/sulfite from hydrogen sulfide (H2S) by RBC membrane-bound eNOS molecules.
H2S and bisulfide (HS–) are both transported very rapidly across the erythrocyte membrane (Jennings, 2013). RBCs typically carry significant levels of glutathione. Oxidized glutathione reacts with H2S in the following simple reaction:
GSSG + H2S → GSSH + GSH
This reaction is actually protective against acute poisoning by excessive H2S exposure, as it has been shown that pretreatment of mice with GSSG protects them from an otherwise lethal dose of sodium sulfide (Smith and Abbanat, 1996). However, it also suggests that RBCs may be able to exploit endogenously produced hydrogen sulfide gas to both reduce oxidized glutathione and synthesize sulfite using eNOS enzymatically, catalyzed by sunlight. The two highly conserved cysteine residues of eNOS become glutathionylated as a modification that enables binding to caveolae and turns off NO synthesis (Duan and Kwan, 2011).
Neutrophils exposed to hydrogen sulfide gas under oxidative conditions produce sulfite, and they are absolutely dependent on NADPH oxidase to do so (Mitsuhashi et al., 2005). Neutrophils also are known to express eNOS (deFrutos et al., 2001), so their synthesis of sulfite could also be catalyzed by eNOS in collaboration with NADPH oxidase, as has been proposed for RBCs. This suggests to us that inflammation, e.g., within the rheumatoid joint, which induces both the expression of reactive oxygen species and the infiltration of neutrophils into the artery wall, may be a process that is needed to renew sulfate supplies to the vasculature (Wright et al., 2010). This is yet another way the body provides the sulfate necessary to maintain water structure both locally and globally.
Cobalamin as a Regulatory Agent
In this section, we first propose that cobalamin (vitamin B12) has a catalytic role in the synthesis of sulfate by eNOS. We then discuss environmental factors that we believe are disrupting the supply of cobalamin to the body, mainly glyphosate, metformin and a strict vegan diet. Finally, we show how the metabolic effects of the loss of cobalamin orchestrate a well-choreographed systemic response involving multiple organs, which is aimed at maintaining sulfate supplies to the vasculature at the expense of the health of the organs.
Cobalamin’s Critical Role in Sulfate Synthesis
Cobalamin is an essential B vitamin with several critical roles in the body. However, we argue here that there is yet another role that has been to date overlooked by the research community. Cobalamin is active in two known forms: adenosylcobalamin and methylcobalamin. However, it is recognized that cobalamin can also conjugate with glutathione. In fact, studies predict that aquacobalamin rapidly reacts with reduced glutathione, irreversibly, to form glutathionylcobalamin (Xia et al., 2004). Early studies that investigated this reaction found that the eventual reaction product was sulfitocobalamin, through an unspecified subsequent reaction sequence (Pezacka et al., 1990, Farquharson and Adams, 1977). Intriguingly, the structure of the eNOS molecule is such that there is a large cavity where the bulky cobalamin molecule should fit perfectly (Wheatley, 2007). Thus, the observations from these published papers can be pieced together to propose that eNOS binds to glutathionylcobalamin. This complex secures a sulfane-sulfur atom, likely from an ambient hydrogen sulfide molecule, which then gets oxidized to SO2/sulfite via the superoxide that is released from eNOS when it is in its uncoupled state. In this way, cobalamin becomes a central player in the sulfate balance and, thus, water structuring throughout the body.
Environmental Factors Leading to Cobalamin Deficiency
Cobalamin is a crucial vitamin for health, even though it only catalyzes a few reactions. Cobalamin deficiency is widespread, with about 20% of the US population being at or below the critical symptomatic threshold of 200pmol/L (Allen, 2008). This is due in part to the complex process involved in cobalamin absorption, which often gets disrupted. Plants neither require nor produce cobalamin, so a vegan diet, becoming increasingly popular in the United States, is likely to be a risk factor for cobalamin deficiency (Antony, 2003). The manifestations of cobalamin deficiency are complex, but extremely interesting. They reflect the intricacies of biological control mechanisms whereby seemingly diverse symptoms orchestrate a higher-level plan that works a “work-around” solution. This is necessitated when the mechanisms that typically synthesize sulfate from sulfane sulfur and hydrogen sulfide are derailed.
Vitamin B12 is a large molecule that is sensitive to degradation in acidic environments. These features make it very difficult to deliver cobalamin to the tissues. The process of cobalamin transport through the gut and past the gut lining is complex. It begins with binding of dietary cobalamin to the protein haptocorrin, secreted by the salivary glands in the oral cavity. Binding to haptocorrin protects B12 from degradation in the acidic environment of the stomach. Pancreatic proteases digest the haptocorrin in the upper intestine. Parietal cells in the stomach produce intrinsic factor, which enters the upper intestines and then binds to the cobalamin following its release from haptocorrin. Without intrinsic factor, only 1% of the cobalamin will be absorbed.
Metformin is a very popular drug widely used to help control blood sugar levels in patients suffering from Type II diabetes. It has become clear that a side effect of long-term metformin therapy is cobalamin deficiency (Kibirige and Mwebaze, 2013). Studies have shown that metformin is effective at reducing blood sugar even when it is administered in a delayed-action formulation that stays in the gut and never even reaches the liver, much less the general circulation (Song, 2016). Part of metformin’s effects on blood sugar is likely due to disruption of cobalamin absorption in the gut, with cobalamin deficiency leading to suppressed hepatic release of glucose from glucagon stores (Ebara et al., 2001, Ebara et al., 2008).
Glypohosate is the active ingredient in the pervasive herbicide, Roundup®. It is the most used herbicide in the world: its usage rate has increased dramatically over the past two decades, in step with the dramatic growth in GMO Roundup-Ready crops such as corn, soy, canola and sugar beets (Benbrook, 2016, Swanson et al., 2014). Glyphosate’s manufacturer, Monsanto, has long maintained that glyphosate is a very safe herbicide with little risk to human health from exposure. Glyphosate has however been found to be present as a contaminant in many common foods, and independent researchers are finding evidence that it is considerably more toxic than the regulators have been led to believe (Séralini et al., 2014, Bonfanti et al., 2018, Santovito et al., 2018).
The most common cause of vitamin B12 deficiency in developed countries is impaired absorption due to a loss of gastric intrinsic factor. Surprisingly, glyphosate is able to diffuse passively across lipid membranes, i.e., across cell plasma membranes, as demonstrated in an elegant set of experiments conducted by B.N. (Ehrl et al., 2018). The ability of glyphosate to cross cell membranes depends critically on the acidity of the medium. Glyphosate’s membrane permeability increases by an order of magnitude at pH 4.1 (mildly acidic), compared to a neutral pH of 7.0. This is an important point when considering glyphosate exposure in the stomach lumen, where the pH is extremely low (1.5 to 3.5). Given this, one can predict that the parietal cells in the stomach will be especially susceptible to dietary glyphosate exposure. One of the important roles of parietal cells is to release intrinsic factor, which is essential for cobalamin transport across the gut wall (Festen, 1991).
Furthermore, cobalamin depends upon cobalt as a cofactor. A study of cows exposed to glyphosate examined blood levels of a suite of minerals, and found alarmingly low levels of just two minerals: cobalt and manganese. Both were at levels well below the minimum of the normal range in all cows at eight different farms (Krüger et al., 2013).
The cobalt atom in cobalamin is housed in the center of a corrin ring, which forms the core of the cobalamin molecule. Corrin is assembled from four molecules of pyrrole, and pyrrole synthesis has been shown to be disrupted by glyphosate. Glyphosate blocks the enzyme δ-aminolevulinic acid synthase, the first step in the synthesis of the pyrrole ring (Kitchen et al., 1981). It likely does this through substrate inhibition, acting as an analogue of glycine.
In addition, eNOS itself is sensitive to glyphosate toxicity. It contains a heme group, which, like the corrin ring, is composed of pyrrole units whose synthesis would be suppressed by glyphosate. Furthermore, eNOS is an orphan member of the cytochrome P450 (CYP) family of enzymes (Gorren and Mayer, 2007): a study of rats showed that liver CYP enzymes were severely suppressed by glyphosate (Hietanen et al., 1983). RBCs express the L-type amino acid transport protein LAT1 (Mann Dosier et al., 2017), which has been shown to actively take up glyphosate into cells (Xu et al., 2016). Glyphosate circulating in the serum would be taken up by RBCs along glycine transport channels, and RBCs have a major need for glycine as a precursor to heme synthesis for their hemoglobin as well as for their eNOS and other heme-dependent enzymes. Finally, eNOS depends on both zinc and iron (in heme) as catalysts, and glyphosate has been shown to chelate these two cations, as well as cobalt, making them unavailable (Mertens et al., 2018). Sulfite oxidase, the enzyme that converts sulfite to sulfate, can also be expected to be impaired by glyphosate due to potential chelation of its cofactor molybdenum, as well as a dependency on heme whose synthesis is suppressed by glyphosate, as previously described.
Consequences of Cobalamin Deficiency
If, as we hypothesize here, eNOS’s synthesis of sulfate depends critically on cobalamin, then cobalamin deficiency can be predicted to lead to sulfate deficiency over time. What is interesting is that the other enzymatic reactions that cobalamin catalyzes, such as methylmalonyl CoA mutase and methionine synthase, involve critical pathways which, when impaired due to cobalamin deficiency, initiate major adjustments in the way the body manages to obtain adequate sulfate. These combined effects of cobalamin and sulfate deficiency initiate a broad range of compensatory changes in the body, many of which are highly symptomatic and, we suggest, incorrectly considered to be unrelated pathologies. These changes are, in brief:
• Sulfur-fixing bacteria proliferate, predominantly in the large bowel, but also occasionally in the small bowel;
• Dietary sulfur, sulfomucins, sulfated hormones, and other compounds are preferentially converted by these bacteria into H2S through well-established pathways (Carbonero et al., 2012);
• These same bacteria also harvest sulfate from bile salts, converting it to H2S (Carbonero et al., 2012);
• The cumulative production of H2S (Jung and Jeong, 2014) disrupts the gut barrier (Medani et al., 2010, Ijssennagger et al., 2016), and H2S
produced in the gut diffuses into circulation to act as substrate for eNOS to generate
SO2. This is transformed as previously described to biologically active sulfate, thus restoring systemic sulfate stores;
• Taurine, stored in the brain and heart, is released in the context of cobalamin deficiency and consequent NMDA receptor activation (Menéndez et al., 1993);
• Circulating taurine can bind to bile salts in the liver, after which it can be converted to sulfate in the digestive tract with assistance from the gut microbes (Carbonero et al., 2012).
Cobalamin deficiency leads to peripheral nerve injury and subsequent Wallerian degeneration of peripheral nerves, whereby the ends of axons distal to the site of injury begin to disintegrate. This involves upregulation of cystathionine beta synthase (CBS) and increased production of H2S, contributing to the H2S pool for vascular oxidation. Further, myelin degradation products, including cysteine and sulfatide, may then be acted upon by CBS, cystathionine γ‐lyase (CSE) and cysteine dioxygenase (CDO) to generate additional H2S (Jung and Jeong, 2014).
All of these adaptations are problematic in terms of health, because they can lead to several pathologies such as; dysbiosis and small intestinal bacteria overgrowth (SIBO) (Banik et al., 2016), and inflammatory bowel diseases such as Crohn’s (Arijs et al., 2013) and ulcerative colitis (Ijssennagger et al., 2016, Zhu et al., 2016, Coutinho et al., 2017). In addition, there may be systemic symptoms of high H2S, including hypotension (Betowski, 2004) and bradycardia (Liu et al., 2011); concentration and memory impairment (Rosenegger et al., 2004, Tvedt et al., 1991, Eto et al., 2002); rheumatic conditions (Muniraj et al., 2017), severe neuropathy, dementia, and arterial damage inducing thrombosis and cardiovascular disease, among other possible issues. Further, the oxidative conditions created by uncoupled eNOS for conversion of H2S to SO2 also increase the risk of thrombosis and cardiovascular disease, neurological disorders, and many other conditions associated with excess oxidative stress.
Within this model, these pathologies are unfortunate side effects of our bodies’ attempts to generate sulfate, compensate for low cobalamin, and ultimately maintain adequate EZ water within tissue and the blood stream. Maintenance of EZ water can only happen when sulfate is in sufficient supply.
Since sulfate deficiency impairs the ZP of RBCs, another adaptive biological compensation is to reduce the number of RBCs in circulation. Macrophages need adequate sulfate to clear the DNA in the nuclei that RBCs release as they mature (Bratosin et al., 1998). The RBC population as a whole needs to be reduced in order to protect from possible agglutination due to insufficient negative charge and EZ water as a consequence of cholesterol sulfate deficiency in their membranes, as described in section 5 above.
Regulatory Mechanisms to Maintain Vascular Health at the Expense of the Heart and Liver
With these concepts in mind, it is now possible to examine the diverse consequences of cobalamin deficiency as it applies to both EZ water formation and related pathologies, schematized in Figure 1. Throughout this discussion it will be important to keep in mind the fact that anything compromising sulfate production will ultimately have a negative impact on EZ water formation throughout the body. Cobalamin is essential for the synthesis of methionine from homocysteine. When this pathway is disrupted, methylation capacity is severely impaired, and homocysteine accumulates to unhealthy levels. Since DNA synthesis depends on methyl groups, cobalamin deficiency causes a reduced proliferation rate of nascent RBCs, resulting in macrocytic anemia. At the same time, homocysteine induces an inflammatory cascade in the artery wall, which causes the release of superoxide to support the oxidation of the sulfur in homocysteine to sulfate, as discussed in a paper by McCully published in 2011 (McCully, 2011). Thus, homocysteine can partially compensate for the loss of eNOS-derived sulfate, but at a severe cost, as inflammation drives cardiovascular disease (Golia et al., 2014).
Vitamin B12 deficiency leads to increased cholesterol uptake and increased cholesterol synthesis in adipocytes. Through the mechanism of epigenetics, genes for proteins involved in cholesterol synthesis and uptake are upregulated as a consequence of hypomethylation of their promoter regions (Adaikalakoteswari et al., 2015). These authors wrote: “Our clinical observations show strong associations of vitamin B12 deficiency with BMI, triglycerides, and total cholesterol, and our in vitro studies in adipocytes show that vitamin B12-deficient conditions induce de novo cholesterol biosynthesis.” (Adaikalakoteswari et al., 2015, p. 10). Epicardial adipose tissue (EAT) accounts for 20% of the total weight of the heart, and obesity is associated with an increase in the amount of EAT (Golia et al., 2014). EAT is infiltrated with macrophages that penetrate the tissue in response to inflammatory signals. In patients with cardiovascular disease, EAT produces higher levels of atherogenic and inflammatory cytokines (Baker et al., 2006). We hypothesize that, as proposed in Seneff et al. (2015), the increased cholesterol storage in fat cells accumulating within cardiovascular arteries provides a ready supply of cholesterol waiting to become cholesterol sulfate once sulfate becomes available.
Glycine is a source of methyl groups via the glycine cleavage system (Kikuchi, 1973). At least for pigs, it appears that cobalamin deficiency suppresses the ability to use methyl groups derived from glycine to synthesize choline, and that this leads to severe fatty liver disease under conditions of dietary choline deficiency (Johnson et al., 1955). Choline deficiency due to insufficient dietary sources along with impaired cobalamin absorption could be a contributory factor in the epidemic we are seeing today in non-alcoholic fatty liver disease (NAFLD) (Corbin and Zeisel, 2012). NAFLD is the most common liver condition worldwide, affecting nearly one in three people in the industrialized world (Smith and Abbanat, 1996). Deficiencies in choline and cobalamin could be especially relevant in the case of a vegan diet, which is generally depleted in both nutrients. A study on rats noted that cobalamin deficiency led to an increase in plasma glycine and serine levels (Ebara et al., 2001): this could be explained by a suppression of the glycine cleavage system, which would also explain the results in the pig study above. The excess bioavailability of glycine due to impaired methylation pathways could be advantageous for heme synthesis, since glycine is a substrate for pyrrole ring synthesis. The heme is needed for the enzymes eNOS and sulfite oxidase involved in synthesizing sulfate from H2S.
Another curious aspect of cobalamin deficiency is that it impairs the adenylyl cyclase system in the liver, leading to a reduction in the synthesis of glucose from glycogen stores in response to hormonal stimuli (Ebara et al., 2008). It may also be a practical response, because the tissues are less able to take up glucose and store it in the extracellular matrix GAGs because of the sulfate deficiency problem. This makes glucose more dangerous as a glycating agent. At the same time, the lipid particles suspended in the blood, as well as the RBCs and platelets, are more susceptible to glycation damage from glucose because they do not have an adequate shield of gelled water surrounding their membranes due to insufficient membrane-bound sulfate.
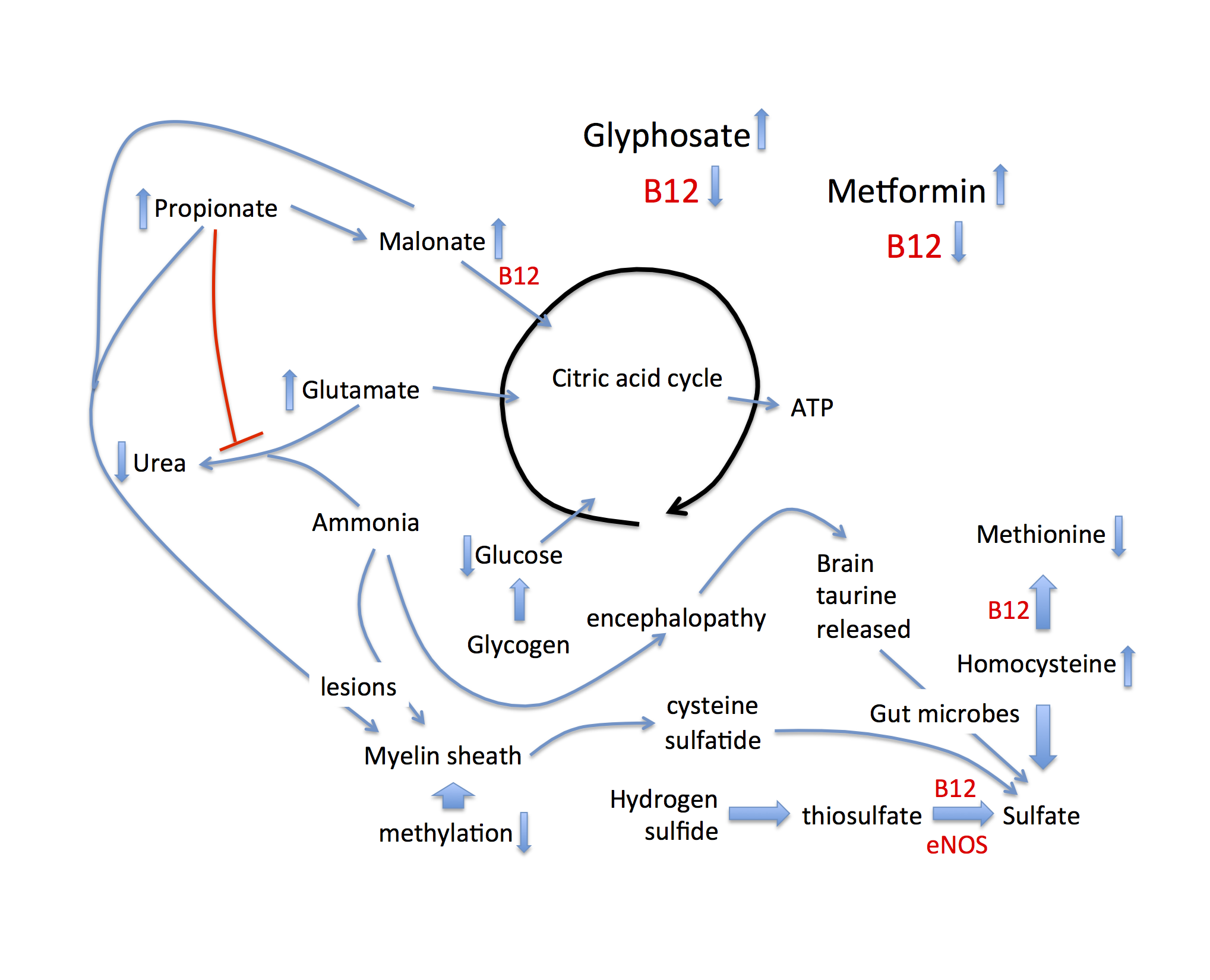
Figure 1: Schematic of metabolic pathways disturbed by B12 deficiency, that lead to alternative approaches to maintaining sulfate supplies to the tissues, associated with disease. Both glyphosate and metformin suppress B12 bioavailability.
Stealing Taurine from the Brain for Sulfate Synthesis by Gut Microbes
Cobalamin also catalyzes an important reaction that converts malonate into succinate, feeding into the citric acid cycle to produce ATP. A precursor to malonate is propionate, a short chain fatty acid produced by the gut microbes in the process of metabolizing branched chain amino acids, fatty acids, cholesterol and sugars via fermentation (Banerjee et al., 1999). A consequence of this blockage is that both propionate and methylmalonate accumulate to high levels. Both are problematic in interesting ways. Propionate interferes with the detoxification of ammonia in the liver. Ammonia detoxification to urea involves a pathway that begins with N-acetylglutamate as substrate.
Acetyl-CoA is a substrate for N-acetylglutamate synthase, and propionyl-CoA acts as a competitive inhibitor of this enzyme (Rabier et al., 1979, Rabier et al., 1986). Consequently, both ammonia and glutamate accumulate to toxic levels. Both have adverse effects on the brain, leading to a chronic low-grade encephalopathy and neurotoxicity. Related to this, rats fed a diet that was high in fermentable carbohydrates exhibited behaviors characteristic of anxiety and aggression that were associated with high levels of propionate in the caecum. This was a consequence of fermentation by the gut microbes (Hanstock et al., 2004). The excess glutamate that is spared from processing through the urea cycle can be channeled into the citric acid cycle (see Figure 1), providing an alternative source of fuel, given that propionate is no longer metabolized via the citric acid cycle. In response to cobalamin deficiency, stored taurine in the brain can be mobilized as a resource to boost sulfate levels, systemically.
Discussions (Seneff et al. 2013) indicate that ammonia has a very high pKa and is therefore one of the few bioavailable molecules that can substitute for taurine as a buffer to support the high pH of the mitochondrial matrix (Hansen et al., 2010). This allows astrocytes and neurons to release their stored taurine (Albrecht et al., 1994), which can then be transported to the gut microbes conjugated to bile acids. While taurine is a very stable molecule that human cells are unable to further metabolize, the gut microbes are able to oxidize taurine to sulfate, thus supplying sulfate to the host. The enzyme in Escherichia coli that oxidizes taurine to sulfite, taurine dioxygenase, depends upon alpha-keto glutarate as a reducing agent (Van der Ploeg et al., 2001, Eichhorn et al., 1997). Thus, the increased bioavailability of glutamate due to suppression of the urea cycle consequential to cobalamin deficiency facilitates taurine oxidation to sulfite by E. coli. NMDA activation induces the release of taurine from neurons (Menéndez et al., 1993). An in vitro study on rat hippocampal slices revealed that glyphosate induces neurotoxicity by a mechanism that involves activation of NMDA receptors and subsequent calcium uptake and increased release of glutamate, a neurotoxin, into the synaptic cleft (Cattani et al., 2014). Decreases in glutathione content and increases in lipoperoxidation were indicators of oxidative stress.
A transcriptome analysis from 2013 of Escherichia coli protein expression in response to glyphosate exposure revealed that many proteins involved in taurine transport were down-regulated (Lu et al., 2013). Taurine transporter subunits tauA, tauB, and tauC, panC (a taurine transport system permease protein), ompF (an ATP-binding component of the taurine transport system), and ompT (a periplasmic protein of the taurine transport system) were all sharply downregulated by E. coli in response to glyphosate exposure. OmpF was downregulated by a factor of 11, and it was among the most downregulated of all the downregulated proteins. This strongly suggests that glyphosate disrupts the metabolism of taurine into inorganic sulfate by microbes.
Taurine-conjugated bile acids (taurocholate) have been identified as a risk factor for colon cancer, and as a potential explanation for the link between a diet high in animal-based foods and colon cancer (Ridlan et al., 2016). This was attributed to the conversion of sulfur-containing amino acids to H2S by sulfur-reducing bacteria such as Desulfovibrio and Bilophila wadsworthia. In particular, B. wadsworthia are notable for their ability to process taurine into H2S (Carbonero et al., 2012). A diet high in milk and milk fat led to over-representation of B. wadsworthia in the mouse gut, likely due to an overabundance of taurine-conjugated bile acids (Devkota et al., 2012). Excessive levels of H2S corrode the gut lining and induce an inflammatory response leading to DNA damage, while the microbially metabolized bile acids cause degradation of tumor-suppressing proteins such as P53 (Qiao et al., 2001). It is here hypothesized that these and other H2S-producing microbes flourish in an environment where common commensals such as E. coli are unable to process taurine and other reduced-sulfur sources into sulfate.
Destruction of the Myelin Sheath
Propionate and malonate (as methylmalonic acid), as well as ammonia, all of which are elevated in response to cobalamin deficiency, are major disruptors of the myelin sheath surrounding nerve fibers. They cause the myelin to become much more fragile and easily attacked by immune cells (Braissant et al., 2013, Kristensen et al., 1993). The myelin is likely more accessible to immune attack because of a destructuring of the surrounding water due to insufficient sulfate in the glycoproteins and glycolipids that surround the fatty sheath. Erosion of the myelin sheath may be the reason for an association between cobalamin deficiency and Alzheimer’s disease (McCaddon et al., 2002). The major protein in the myelin sheath is called myelin proteolipid protein peptide, and it is rich in cysteine residues, a potential source for sulfate. But, perhaps even more convenient as a direct source of sulfate is the sulfatide, a sulfated glycolipid normally present in high amounts in the myelin sheath. Sulfatide levels in the brains of Alzheimer’s patients plummet very early in the disease process (Han et al., 2002). These authors found an astonishing 93% depletion of sulfatide already in the very early stages of Alzheimer’s disease. A study comparing Alzheimer’s patients to controls showed that the Alzheimer’s patients had significantly elevated levels of methylmalonic acid (p = 0.0097), which was associated with cobalamin deficiency (Kristensen et a., 1993). We hypothesize that the myelin sheath is attacked in order to retrieve sulfate from the sulfatide to help restore sulfate levels in the circulation.
In addition to dementia, cobalamin deficiency is also linked to multiple other neurological diseases, including myelopathy, peripheral neuropathy and optic neuropathy (Saperstein and Barohn, 2002). Elevated serum methylmalonic acid and homocysteine are diagnostic features. It is likely that these conditions are a consequence of a stripping of the sulfatide and sulfur-enriched proteins in the myelin sheath of peripheral neurons.
Conclusion
In this paper we have proposed that the sulfate molecule plays a unique role within biology as the source of the negative charge needed for the formation and maintenance of EZ water in living system. We have described unique roles for eNOS and cobalamin within the system of ongoing sulfate production and regulation. It is our assertion that several diverse and seemingly unrelated pathological processes might have a common origin as compensatory processes to reestablish sulfate sufficiency for EZ water maintenance. We also find that pathologies traditionally associated with cobalamin deficiency can be best understood through the impact that that deficiency has on sulfate metabolism, and the compensatory changes that this causes.
While our proposed roles for eNOS and cobalamin in this model are unique, we believe the circumstantial evidence presented here is compelling and warrants further research. If our model of EZ water production and maintenance through the interface of water and sulfated molecules is correct, it has wide implications in health and medicine. Further, if we are correct that glyphosate has a broad range of detrimental impacts on sulfate production, it adds to the growing body of evidence that its widespread use requires renewed scrutiny and regulation.
Science progresses to a large extent by speculative hypotheses that, through further investigation, transform into established fact. It is our hope that these ideas might provide a foundation for understanding the formation and maintenance of interfacial water in the body, and eventually progress to a set of therapeutic strategies for addressing the wide-ranging pathologies that might be related to its loss.
Discussion with Reviewers
1. In your opinion what agents will be suggested in the nearest future to increase the exclusion zone size and health of biological tissues?
If we are correct that the maintenance of health is dependent upon the proper utilization of sulfur and its metabolites, then those compounds that play a role in sulfur metabolism in general, and hydrogen sulfide metabolism in particular, would be expected to have uniquely beneficial roles in health maintenance. Many examples support this idea.
Coenzyme Q10 is an essential component of mitochondrial electron transfer. Much research has shown its beneficial role in enhancing energy production. A lesser known role of CoQ10, though, is as an essential cofactor in the activity of sulfite: quinone reductase (SQR), where it facilitates conversion of H2S to SO3–, thus feeding into the SO4-2 pool via the SUOX enzyme.
There are several other nutrients that play a lesser-known role in H2S and SO4-2 metabolism and distribution around the body, including vitamin D, vitamin C, curcumin, resveratrol, and others. These are, perhaps not coincidentally, among the nutrients with the strongest health-supporting evidence. While their role in sulfur metabolism is virtually never mentioned in articles on their health benefits, we propose that it may be as significant as their antioxidant and other properties.
In this respect, it is our prediction that agents that might be suggested in the future for increasing the size of the exclusion zone will be agents that contribute to the H2S/SO4-2 cycle in the body we have described in this paper. They will do so in a way that ultimately facilitates production and/or distribution and/or utilization of SO4-2 and its critical role in supplying the electronegative charge to the hydrophilic surfaces within the body.
2. In your opinion what novel physical methods will be suggested in the nearest future to increase the exclusion zone size and health of biological tissues?
To our knowledge, the only physical therapy thus far shown to increase the size of the exclusion zone is the application of infrared light, with Pollack reporting that IR of a wavelength 3000nm having the greatest impact on EZ extension. The scope of the electromagnetic spectrum is astonishing, encompassing 20 orders of magnitude. Given the increasing number of health benefits discovered for very specific frequencies along that spectrum, it is our prediction that electromagnetic medicine will be a growing field of interest. The full EM spectrum is essentially a virtually untapped ocean of potential specific frequencies that might have EZ-extending properties.
Author Contributions
Both authors contributed significantly to all aspects of the paper.
Funding
This research was funded in part by Quanta Computers, Taiwan under the auspices of the Qmulus project.Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
CYP: Cytochrome P450
EVSP: Electrokinetic Vascular Streaming Potential
eNOS: Endothelial Nitric Oxide Synthase
EAT: Epicardial adipose tissue
EZ: Exclusion Zone
FAD: Flavin Adenine Dinucleotide
FMN: Flavin Mononucleotide
GAGs: Glycosaminoglycans
HDL: High Density Lipoprotein
iNOS: Inducible Nitric Oxide Synthase
LDL: Low Density Lipoprotein
NADPH: Nicotinamide Adenine Dinucleotide Phosphate
NO: Nitric Oxide
NAFLD: Non-alcoholic Fatty Liver Disease
ONOO-: Peroxynitrite
SIBO: Small Intestinal Bacterial Overgrowth
SO2: Sulfur Dioxide
BH4: Tetrahydrobiopterin
ZP: Zeta Potential
References
Adaikalakoteswari A, Finer S, Voyias PD, McCarthy CM, Vatish M, Moore J, Smart-Halajko M, Bawazeer N, Al-Daghri NM, McTernan PG, Kumar S, Hitman GA, Saravanan P, Tripathi G (2015). Vitamin B12 insufficiency induces cholesterol biosynthesis by limiting S-adenosylmethionine and modulating the methylation of SREBF1 and LDLR genes. Clin Epigenetics 7: 14.
Albrecht, J.; Bender, A.S.; Norenberg, M.D (1994). Ammonia stimulates the release of taurine from cultured astrocytes. Brain Res 660: 228-232.
Allen LH (2008). How common is vitamin B-12 deficiency? Amer J Clin Nutr 89: 693S-696S.
Antony AC (2003). Vegetarianism and vitamin B-12 (cobalamin) deficiency. Am J Clin Nutr 78: 3-6.
Arijs I, Vanhove W, Rutgeerts P, Schuit F, Verbeke K, De Preter V (2013). Decreased mucosal sulfide detoxification capacity in patients with Crohn’s disease. Inflammatory Bowel Diseases 19: E70-E72.
Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG (2006). Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 5: 1.
Ball P (2008). Water as an active constituent in cell biology. Chemical Reviews 108: 74-108.
Banerjee R, Chowdhury S. Methylmalonyl-CoA mutase. In: Banerjee R, editor (1999). Chemistry and Biochemistry of B12. John Wiley & Sons, Inc.: New York pp. 707-729.
Banerjee SK, Maulik SK (2002). Effect of garlic on cardiovascular disorders: a review. Nutr J 1: 4.
Banik GD, De A, Som S, Jana S, Daschakraborty SB, Chaudhuri S, Pradhan M (2016). Hydrogen sulphide in exhaled breath: a potential biomarker for small intestinal bacterial overgrowth in IBS. J Breath Res 10: 026010.
Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW (2007). Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A 104: 17977-82.
Benbrook CM (2016). Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28: 3.
Bergner EA, Shapiro LJ (1981). Increased cholesterol sulfate in plasma and red blood cell membranes of steroid sulfatase deficient patients. J Clin Endocrinol Metab 53: 221-3.
Betowski J (2004). [Hydrogen sulfide as a biologically active mediator in the cardiovascular system]. [Article in Polish] Postepy Hig Med Dosw (Online) 58: 285-91.
Bonfanti P, Saibene M, Bacchetta R, Mantecca P, Colombo A (2018). A glyphosate micro-emulsion formulation displays teratogenicity in Xenopus laevis. Aquat Toxicol 195: 103-113.
Braissant O, McLin VA, Cudalbu C (2013). Ammonia toxicity to the brain. J Inherit Metab Dis 36: 595-612.
Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, Aminoff D, Montreuil J (1998). Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie 80: 173-95.
Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR (2012). Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol 3: 448.
Cattani D, de Liz Oliveira Cavalli VL, Heinz Rieg CE, Domingues JT, Dal-Cim T, Tasca CI, Mena Barreto Silva FR, Zamoner A (2014). Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: involvement of glutamate excitotoxicity. Toxicology 320: 34-45. doi: 10.1016/j.tox.2014.03.001.
Chai B, Yoo H, Pollack GH (2009). Effect of radiant energy on near-surface water. J Phys Chem B 113: 13953-13958.
Chen Z, Bakhshi FR, Shajahan AN, Sharma T, Mao M, Trane A, Bernatchez P, van Nieuw Amerongen GP, Bonini MG, Skidgel RA, Malik AB, Minshall RD (2012). Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol Biol Cell 23: 1388-98.
Corbin KD, Zeisel SH (2012). Choline metabolism provides novel insights into non-alcoholic fatty liver disease and its progression. Curr Opin Gastroenterol 28: 159-165.
Coutinho CMLM, Coutinho-Silva R, Zinkevich V, Pearce CB, Ojcius DM, Beech I (2017). Sulphate-reducing bacteria from ulcerative colitis patients induce apoptosis of gastrointestinal epithelial cells. Microb Pathog 112: 126-134.
Damadian R (1971). Tumor detection by nuclear magnetic resonance. Science 171: 1151-1153.
Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB (2012). Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 487: 104-108.
Duan DD, Kwan CY (2011). A molecular switch of ”yin and yang”: S-glutathionylation of eNOS turns off NO synthesis and turns on superoxide generation. Acta Pharmacol Sin 32: 415-6.
Duer M, Veis A (2013). Bone mineralization: Water brings order. Nature Materials 12: 1081.
Ebara S, Nakao M, Tomoda M, Yamaji R, Watanabe F, Inui H, Nakano Y (2008). Vitamin B12 deficiency results in the abnormal regulation of serine dehydratase and tyrosine aminotransferase activities correlated with impairment of the adenylyl cyclase system in rat liver. Br J Nutr 99: 503-10.
Ebara S, Toyoshima S, Matsumura T, Adachi S, Takenaka S, Yamaji R, Watanabe F, Miyatake K, Inui H, Nakano Y (2001). Cobalamin deficiency results in severe metabolic disorder of serine and threonine in rats. Biochim Biophys Acta 1568: 111-7.
Ehrl BN, Mogusu EO, Kim K, Hofstetter H, Pedersen JA, Elsner M (2018). High Permeation Rates in Liposome Systems Explain Rapid Glyphosate Biodegradation Associated with Strong Isotope Fractionation. Environ Sci Technol 52: 7259-7268.
Eichhorn E, van der Ploeg JR, Kertesz MA, Leisinger T (1997). Characterization of alpha-ketoglutarate-dependent taurine dioxygenase from Escherichia coli. J Biol Chem 272: 23031-6.
Eto K, Asada T, Arima K, Makifuchi T, Kimura H (2002). Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem Biophys Res Commun 293: 1485-8.
Farquharson J, Adams JF (1977). Conversion of hydroxo(aquo) cobalamin to sulfitocobalamin in the absence of light: a reaction of importance in the identification of the forms of vitamin B12, with possible clinical significance. Am J Clin Nutr 30: 1617-22. doi: 10.1093/ajcn/30.10.1617
Faure C, Tranchant JF, Dufourc EJ (1996). Comparative effects of cholesterol and cholesterol sulfate on hydration and ordering of dimyristoylphosphatidylcholine membranes. Biophys J 70: 1380-90.
Fernandes HP, Cesar CL, Barjas-Castro Mde L (2011). Electrical properties of the red blood cell membrane and immunohematological investigation. Rev Bras Hematol Hemoter 33: 297-301.
Feron O, Dessy C, Moniotte S, Desager JP, Balligand JL (1999). Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J Clin Invest 103: 897-905.
Festen HP (1991). Intrinsic factor secretion and cobalamin absorption. Physiology and pathophysiology in the gastrointestinal tract. Scand J Gastroenterol Suppl 188: 1-7.
Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, Bianchi R, Crisci M, D’Acierno L, Giordano R, Di Palma G, Conte M, Golino P, Russo MG, Calabr R, Calabr P (2014). Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep 16: 435.
Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Russo PE, Riegler L, Bianchi R, Crisci M, Palma GD, Golino P, Russo MG, Calabr R, Calabr P (2014). Adipose tissue and vascular inflammation in coronary artery disease. World J Cardiol 6: 539-54.
Gorren AC, Mayer B (2007). Nitric-oxide synthase: A cytochrome P450 family foster child. Biochim Biophys Acta 1770: 432-445.
Griffith OW, Stuehr DJ (1995). Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol 57: 707-734.
Han X, Holtzman DM, McKeel DW Jr., Kelley J, Morris JC (2002). Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J Neurochem 82: 809-818.
Hansen SH, Andersen ML, Cornett C, Gradinaru R, Grunnet N (2010). A role for taurine in mitochondrial function. J Biomed Sci 17: S23.
Hanstock TL, Clayton EH, Li KM, Mallet PE (2004). Anxiety and aggression associated with the fermentation of carbohydrates in the hindgut of rats. Physiol Behav 82: 357-68.
Hietanen E, Linnainmaa K, Vainio H (1983). Effects of phenoxyherbicides and glyphosate on the hepatic and intestinal biotransformation activities in the rat. Acta Pharmacol Toxicol (Copenh) 53: 103-12.
Hwang SG, Hong JK, Sharma A, Pollack GH, Bahng G (2018). Exclusion zone and heterogeneous water structure at ambient temperature. PLoS ONE 13: e0195057.
Iciek M, Bilska-Wilkosz A, Górny M, Sokołowska-Jeżewicz M, Kowalczyk-Pachel D (2016). The effects of different garlic-derived allyl sulfides on anaerobic sulfur metabolism in the mouse kidney. Antioxidants (Basel) 5: pii: E46.
Ijssennagger N, van der Meer R, van Mil SWC (2016). Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Disease? Trends Mol Med 22: 190-199.
Iwama T, Yamada H, Era S, Sogami M, Andoh T, Sakai N, Kato K, Kuwata K, Watari H (1992). Proton nuclear magnetic resonance studies on water structure in peritumoral edematous brain tissue. Magn Reson Med 24: 53-63.
Jennings ML (2013). Transport of H2S and HS – across the human red blood cell membrane: rapid H2S diffusion and AE1-mediated Cl-/HS- exchange. Am J Physiol Cell Physiol 305: C941-C950.
Johnson BC, Firth J, Mistry SP (1955). Vitamin B12 and choline synthesis from glycine in vivo. Arch Biochem Biophys 54: 467-473.
Jolande J, ed. (1988). Selected Writings. Vol. 28. Princeton University Press: Princeton, New Jersey, USA.
Jung J, Jeong NY (2014). Hydrogen sulfide controls peripheral nerve degeneration and regeneration: a novel therapeutic strategy for peripheral demyelinating disorders or nerve degenerative diseases. Neural Regen Res 9: 2119-21.
Kang ES, Ford K, Grokulsky G, Wang YB, Chiang TM, Acchiardo SR (2000). Normal circulating adult human red blood cells contain inactive NOS proteins. J Lab Clin Med 135: 444-451.
Kerch G (2018). Distribution of tightly and loosely bound water in biological macromolecules and age-related diseases. Int J Biol Macromol 118: 1310-1318.
Kibirige D, Mwebaze R (2013). Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J Diabetes Metab Disord 12: 17.
Kikuchi G (1973) The glycine cleavage system: Composition, reaction mechanism, and physiological significance. Mol Cell Biochem 1: 169-187.
Kitchen LM, Witt WW, Rieck CE (1981). Inhibition of δ-aminolevulinic acid synthesis by glyphosate. Weed Science 29: 571-577.
Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozüyaman B, Schnürch HG, Gödecke A, Weber AA, Robenek M, Robenek H, Bloch W, Rösen P, Kelm M (2006). Red blood cells express a functional endothelial nitric oxide synthase. Blood 107: 2943-2951.
Knowles RG, Moncada S (1994). Nitric oxide synthases in mammals. Biochem J 298: 249-258.
Kristensen MO, Gulmann NC, Christensen JE, Ostergaard K, Rasmussen K (1993). Serum cobalamin and methylmalonic acid in Alzheimer dementia. Acta Neurol Scand 87: 475-81.
Krüger M, Schrödl W, Neuhaus J, Shehata AA (2013). Field investigations of glyphosate in urine of Danish dairy cows. J Environ Anal Toxicol 3: 1-7.
Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG (2003). Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201-9.
Le Bihan D (2007). The “wet mind:” water and functional neuroimaging. Phys Med Biol 52: R57.
Liu WQ, Chai C, Li XY, Yuan WJ, Wang WZ, Lu Y (2011). The cardiovascular effects of central hydrogen sulfide are related to K(ATP) channels activation. Physiol Res 60: 729-38.
Lu W, Li L, Chen M, Zhou Z, Zhang W, Ping S, Yan Y, Wang J, Lin M (2013). Genome-wide transcriptional responses of Escherichia coli to glyphosate, a potent inhibitor of the shikimate pathway enzyme 5-enolpyruvylshikimate-3-phosphate synthase. Mol Biosyst 9: 522-30.
Mann Dosier LB, Premkumar VJ, Zhu H, Akosman I, Wempe MF, McMahon TJ (2017). Antagonists of the system L neutral amino acid transporter (LAT) promote endothelial adhesivity of human red blood cells. Thromb Haemost 117: 1402-1411.
McCaddon A, Regland B, Hudson P, Davies G (2002). Functional vitamin B(12) deficiency and Alzheimer disease. Neurology 58: 1395-9.
McCully KS (2011). Chemical pathology of homocysteine. V. Thioretinamide, thioretinaco, and cystathionine synthase function in degenerative diseases. Ann Clin Lab Sci 41: 301-14.
Medani M, Collins D, Docherty NG, Baird AW, O’Connell PR, Winter DC (2010). Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm Bowel Dis 17: 1620-1625.
Mehta AL, Mehta P, Li D (2000). Nitric oxide synthase in adult red blood cells: Vestige of an earlier age or a biologically active enzyme? J Lab Clin Med 135: 430-431.
Melacini G, Bonvin AM, Goodman M, Boelens R, Kaptein R (2000). Hydration dynamics of the collagen triple helix by NMR. J Mol Biol 300: 1041-9.
Melkumyants AM, Balashov SA, Khayutin VM (1989). Endothelium dependent control of arterial diameter by blood viscosity. Cardiovasc Res 23: 741-747.
Menéndez N, Sols JM, Herreras O, Galarreta M, Conejero C, Martín del Ro R (1993). Taurine release evoked by NMDA receptor activation is largely dependent on calcium mobilization from intracellular stores. Eur J Neurosci 5: 1273-9.
Mertens M, Höss S, Günter Neumann G, Afzal J, Reichenbecher W (2018). Glyphosate, a chelating agent relevant for ecological risk assessment? Environ Sci Pollut Res Int 25: 5298-5317.
Mitsuhashi H, Yamashita S, Ikeuchi H, Kuroiwa T, Kaneko Y, Hiromura K, Ueki K, Nojima Y (2005). Oxidative stress-dependent conversion of hydrogen sulfide to sulfite by activated neutrophils. Shock 24: 529-34.
Morris ME, Levy G (1983). Serum concentration and renal excretion by normal adults of inorganic sulfate after acetaminophen, ascorbic acid, or sodium sulfate. Clin Pharmacol Ther 33: 529-536.
Muniraj N, Stamp LK, Badiei A, Hegde A, Cameron V, Bhatia M (2017). Hydrogen sulfide acts as a pro-inflammatory mediator in rheumatic disease. Int J Rheum Dis 20: 182-189.
Nwose EU (2010). Whole blood viscosity assessment issues I: Extrapolation chart and reference values. N Am J Med Sci 2: 165.
Pezacka E, Green R, Jacobsen DW (1990). Glutathionylcobalamin as an intermediate in the formation of cobalamin coenzymes. Biochem Biophys Res Commun 169: 443-50.
Pizzitutti F, Marchi M, Sterpone F Rossky PJ (2007). How protein surfaces induce anomalous dynamics of hydration water. J Phys Chem B 111: 7584-7590.
Pollack GH, Clegg J (2008). Unexpected Linkage Between Unstirred Layers, Exclusion Zones, and Water. In: Pollack G.H., Chin WC. (eds) Phase Transitions in Cell Biology. Springer: Dordrecht, Netherlands.
Przybylska M, Faber M, Zaborowski A, Swietosawski J, Bryszewska M (1998). Morphological changes of human erythrocytes induced by cholesterol sulphate. Clin Biochem 31: 73-79.
Qiao D, Gaitonde SV, Qi W, Martinez JD (2001). Deoxycholic acid suppresses p53 by stimulating proteosome-mediated p53 protein degradation. Carcinogenesis 22: 957-964.
Rabier D, Briand P, Petit F, Kamoun P, Cathelineau L (1986). Effects of organic acids on the synthesis of citrulline by intact rat liver mitochondria. Biochimie 68: 639-47.
Rabier D, Cathelineau L, Briand P, Kamoun P (1979). Propionate and succinate effects on acetyl glutamate biosynthesis by rat liver mitochondria. Biochem Biophys Res Comm 91: 456-460.
Ridlon JM, Wolf PG, Gaskins HR (2016). Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes 7: 201-15.
Rodriguez WV, Wheeler JJ, Klimuk SK, Kitson CN, Hope MJ (1995). Transbilayer movement and net flux of cholesterol and cholesterol sulfate between liposomal membranes. Biochemistry 34: 6208-6217.
Rogers ME, Williams DT, Niththyananthan R, Rampling MW, Heslop KE, Johnston DG (1992). Decrease in erythrocyte glycophorin sialic acid content is associated with increased erythrocyte aggregation in human diabetes. Clin Sci (Lond) 82: 309-13.
Rosenegger D, Roth S, Lukowiak K (2004). Learning and memory in Lymnaea are negatively altered by acute low-level concentrations of hydrogen sulphide. J Exp Biol 207: 2621-30.
Roux L, Holojda S, Sundblad G, Freeze HH, Varki A (1988). Sulfated N-Linked oligosaccharides in mammalian cells I. Complex-type chains with sialic acids and O-sulfate esters. JBC 263: 8879-8889.
Russo D (2008). The impact of kosmotropes and chaotropes on bulk and hydration shell water dynamics in a model peptide solution. Chem Phys 345: 200-211.
Santovito A, Ruberto S, Gendusa C, Cervella P (2018). In vitro evaluation of genomic damage induced by glyphosate on human lymphocytes. Environ Sci Pollut Res Int 25: 34693-34700.
Saperstein DS, Barohn RJ (2002). Peripheral neuropathy due to cobalamin deficiency. Curr Treat Options Neurol 4: 197-201.
Schneider MJ, Schneider AS (1972). Water in biological membranes: adsorption isotherms and circular dichroism as a function of hydration. J Membr Biol 9: 127-40.
Seneff S, Davidson RM, Lauritzen A, Samsel A, Wainwright G (2015). A novel hypothesis for atherosclerosis as a cholesterol sulfate deficiency syndrome. Theor Biol Med Model 12: 9.
Seneff S, Lauritzen A, Davidson R, Lentz-Marioin L (2012). Is endothelial nitric oxide synthase a moonlighting protein whose day job is cholesterol sulfate synthesis? Implications for cholesterol transport, diabetes and cardiovascular disease. Entropy 14: 2492-2530.
Seneff S, Lauritzen A, Davidson RM, Lentz-Marino L (2013). Is Encephalopathy a Mechanism to Renew Sulfate in Autism? Entropy 15: 372-406.
Sharma A, Adams C, Cashdollar BD, Li Z, Nguyen NV, Sai H, Shi J, Velchuru G, Zhu KZ, Pollack GH (2018). Effect of health-promoting agents on exclusion-zone size. Dose Response 16: 1559325818796937.
Smith RP, Abbanat RA (1996). Protective effect of oxidized glutathione in acute sulfide poisoning. Toxicol Appl Pharmacol 9: 209-217.
Song R (2016). Mechanism of metformin: A tale of two sites. Diabetes Care 39: 187-189.
Srikanth CB, Salimath PV, Nandini CD (2012). Erythrocytes express chondroitin sulphate/dermatan sulphate, which undergoes quantitative changes during diabetes and mediate erythrocyte adhesion to extracellular matrix components. Biochimie 94: 1347-1355.
Stadler AM, Embs JP, Digel I, Artmann GM, Unruh T, Büldt G, Zaccai G (2008). Cytoplasmic water and hydration layer dynamics in human red blood cells. J Am Chem Soc 13: 16852-16853.
Strott CA, Higashi Y (2003). Cholesterol sulfate in human physiology: what’s it all about? J Lipid Res 44: 1268-78.
Swanson NL, Leu A, Abrahamson J, Wallet B (2014). Genetically engineered crops, glyphosate and the deterioration of health in the United States of America. J Org Systems 9: 6-37.
Syed DK, Rizvi I (2013). Erythrocyte membrane bound and plasma sialic acid during aging. Biologia 68: 762-765.
Szent- Györgyi A (1956). Bioenergetics. Science 124: 873-875.
Séralini GE, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D, de Vendômois JS (2014). Republished study: Long-term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Environ Sci Eur 26: 14.
Takahashi S, Mendelsohn ME (2003). Calmodulin-dependent and -independent activation of endothelial nitric-oxide synthase by heat shock protein 90. J Biol Chem 278: 9339-44.
Takahashi T, Suzuki T (2012). Role of sulfatide in normal and pathological cells and tissues. J Lipid Res 53: 1437-1450. doi: 10.1194/jlr.R026682.
Trivedi DP, Hallock KJ, Bergethon PR (2013). Electric fields caused by blood flow modulate vascular endothelial electrophysiology and nitric oxide production. Bioelectromagnetics 34: 22-30.
Tvedt B, Skyberg K, Aaserud O, Hobbesland A, Mathiesen T (1991). Brain damage caused by hydrogen sulfide: a follow-up study of six patients. Am J Ind Med 20: 91-101.
Unger E, Littlefield J, Gado M (1988). Water content and water structure in CT and MR signal changes: possible influence in detection of early stroke. Am J Neuroradiol 9: 687-691.
Van der Ploeg JR, Eichhorn E, Leisinger T (2001). Sulfonate-sulfur metabolism and its regulation in Escherichia coli. Archives of Microbiology 176: 1-8.
Vink H, Duling BR (2000). Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol-Heart Circ Physiol 278: H285-H289.
Wheatley C (2007). Cobalamin in inflammation III glutathionylcobalamin and methylcobalamin/adenosylcobalamin coenzymes: the sword in the stone? How cobalamin may directly regulate the nitric oxide synthases. J Nutr Environ Med 16: 212-226.
Wright HL, Moots RJ, Bucknall RC, Edwards SW (2010). Neutrophil function in inflammation and inflammatory diseases. Rheumatology 49: 1618-1631.
Xia L, Cregan AG, Berben LA, Brasch NE (2004). Studies on the formation of glutathionyl-cobalamin: any free intracellular aquacobalamin is likely to be rapidly and irreversibly converted to glutathionylcobalamin. Inorg Chem 43: 6848-57.
Xu J, Li G, Wang Z, Si L, He S, Cai J, Huang J, Donovan MD (2016). The role of L-type amino acid transporters in the uptake of glyphosate across mammalian epithelial tissues. Chemosphere 145: 487-94.
Yoo H, Paranji R, Pollack GH (2011). Impact of hydrophilic surfaces on interfacial water dynamics probed with NMR spectroscopy. J Phys Chem Lett 2: 532-536.
Zhu S, Li S, Chen P, Guan R, Gao L (2016). The relationship between expression of endogenous cystathionine beta-synthase and hydrogen sulfide in rectal mucosa tissue and the activeness of ulcerative colitis. Chinese Journal of Postgraduates of Medicine 39: 1064-1068.
Zou M-H, Shi C, Cohen RA (2002). Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J Clin Invest 109: 817-826. .
Zweier JL, Chen C-A, Druhan LJ (2011). S-Glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antioxid Redox Signal 14: 1769-1775.
de Frutos, Sánchez de Miguel L, Farré J, Gómez J, Romero J, Marcos-Alberca P, Nuñez A, Rico L, López-Farré A (2001). Expression of an endothelial-type nitric oxide synthase isoform in human neutrophils: modification by tumor necrosis factor-alpha and during acute myocardial infarction. J Am Coll Cardiol 37: 800-7.
